JEE Advance - Chemistry (2013 - Paper 1 Offline - No. 12)
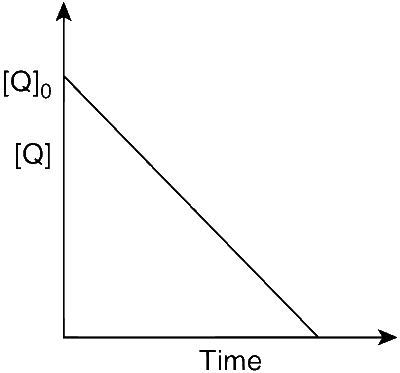
In the reaction, P + Q $$\to$$ R + S, the time taken for 75% reaction of P is twice the time taken for 50% reaction of P. The concentration of Q varies with reaction time as shown in the figure. The overall order of the reaction is
2
3
0
1
Explanation
This is a first-order reaction because t75% = 2 $$\times$$ t50%. The graph shows that the order with respect to Q is 0, so we can write the rate expression as
Rate = k[P]1[Q]0
Comments (0)


