JEE Advance - Chemistry (2013 - Paper 1 Offline - No. 11)
Consider the following complex ions : P, Q and R.
$$P = {[Fe{F_6}]^{3 - }}$$, $$Q = {[V{({H_2}O)_6}]^{2 + }}$$ and $$R = {[Fe{({H_2}O)_6}]^{2 + }}$$
The correct order of the complex ions, according to their spin-only magnetic moment values (in B.M.) is
R < Q < P
Q < R < P
R < P < Q
Q < P < R
Explanation
The electronic configurations of the central metal ions are as follows:
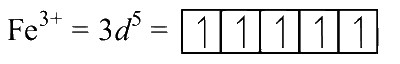
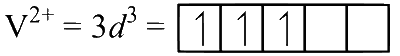
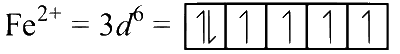
The number of unpaired electrons is P = 5, Q = 3 and R = 4. From the relation $$\mu = \sqrt {n(n + 2)} $$, where n is the number of unpaired electrons, we have the order of spin only magnetic moment as Q < R < P.
Comments (0)


