JEE Advance - Chemistry (2012 - Paper 1 Offline - No. 8)
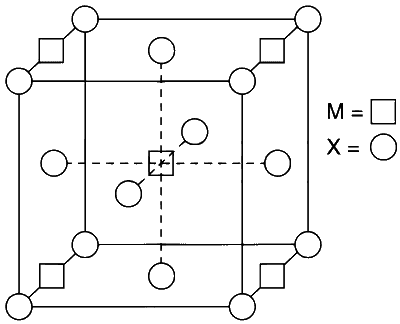
A compound MpXq has cubic close packing (ccp) arrangement of X. Its unit cell structure is shown below. The empirical formula of the compound is
MX
MX2
M2X
M5X14
Explanation
8 X atoms present at the corners.
Atoms contribute to 1 unit cell $$ = {1 \over 8} \times 8 = 1$$
6 X atoms present at the face centres.
Atoms contribute to 1 unit cell $$ = 6 \times {1 \over 2} = 3$$
Total X atoms $$ = 3 + 1 = 4$$
4 M atoms present at edge centres.
Atoms present in 1 unit cell $$ = 4 \times {1 \over 4} = 1$$
1 M atom present at body centre and it contribute completely to 1 unit cell.
Thus, total M atoms in one unit cell $$ = 1 + 1 = 2$$
Ratio is $$M:X::2:4::1:2$$
Thus, empirical formula is $$M{X_2}$$.
Comments (0)


