JEE Advance - Chemistry (2012 - Paper 1 Offline - No. 19)
The substituents R1 and R2 for nine peptides are listed in the table given below. How many of these peptides are positively charged at pH = 7.0 ?
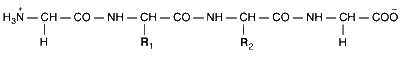
| Peptide | $${R_1}$$ | $${R_2}$$ |
|---|---|---|
| I | H | H |
| II | H | $$C{H_3}$$ |
| III | $$C{H_2}COOH$$ | H |
| IV | $$C{H_2}CON{H_2}$$ | $${(C{H_2})_4}N{H_2}$$ |
| V | $$C{H_2}CON{H_2}$$ | $$C{H_2}CON{H_2}$$ |
| VI | $${(C{H_2})_4}N{H_2}$$ | $${(C{H_2})_4}N{H_2}$$ |
| VII | $$C{H_2}COOH$$ | $$C{H_2}CON{H_2}$$ |
| VIII | $$C{H_2}OH$$ | $${(C{H_2})_4}N{H_2}$$ |
| IX | $${(C{H_2})_4}N{H_2}$$ | $$C{H_3}$$ |
Answer
4
Explanation
For basic amino acids with pH > 7, peptides will exist as cations. For example, when the substituents are basic, that is R1 = CH2CONH2 and R2 = (CH2)4NH2 or when R1 = (CH2)4NH2 and R2 = (CH2)4NH2 or when R1 = CH2OH and R2 = (CH2)4NH2 or when R1 = (CH2)4NH2 and R2 = CH3.
Comments (0)


