JEE Advance - Chemistry (2012 - Paper 1 Offline - No. 18)
Explanation
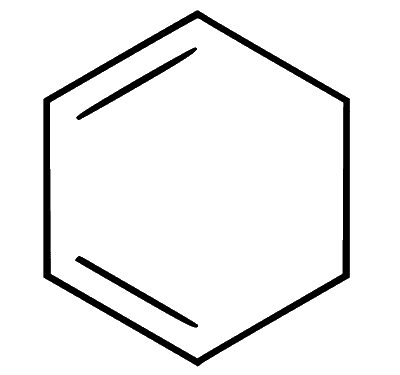
(a) The compound is monocyclic but non-planar due to presence of two $s p^3$ hybridised carbon atoms. As a result, delocalisation of pi $(\pi)$ electrons (or a conjugate system) is not possible. The compound is non-aromatic.
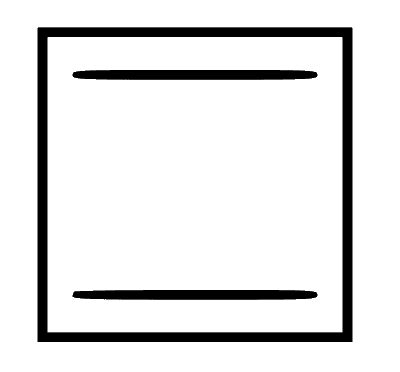
(b) The compound is monocyclic and planar. Carbon atoms are $s p^2$ hybridised and the pi $(\pi)$ electrons are conjugated, i.e., there is delocalisation of pi $(\pi)$ electrons. It follows $4 n$$\pi$ electron system with $4 \pi$ electrons. This makes the compound anti-aromatic and least stable.
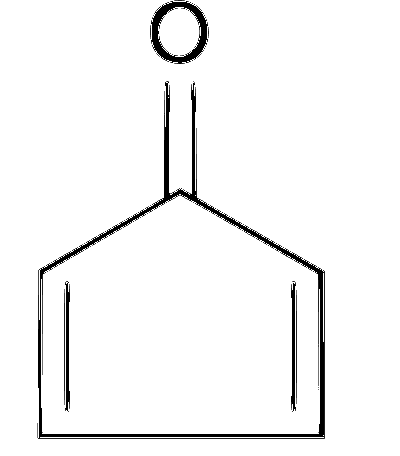
(c) The compound is monocyclic and planar. The carbons including carbonyl carbon are $s p^2$ hybridised. There is no extended delocalisation of pi ( $\pi$ ) electrons (due to exocyclic carbonyl double bond). It also follows $4 n \pi$ electron system with $4 \pi$ electrons inside the ring. This makes compound anti-aromatic and least stable.
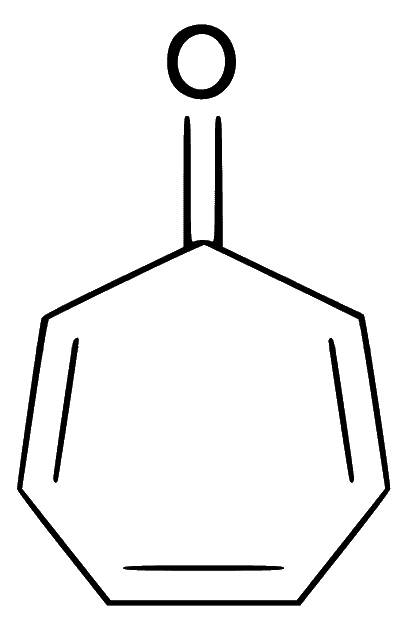
(d) The compound is monocyclic and planar. The all carbons including carbonyl carbons are $s p^2$ hybridised. Though, there is no extended delocalisation of electron inside the ring, but ring has $(4 n+2) \pi$ electrons, i.e., $6 \pi$ electrons. This makes compound aromatic and most stable.
Comments (0)


