JEE Advance - Chemistry (2012 - Paper 1 Offline - No. 17)
For an ideal gas, consider only P-V work in going from an initial state X to the final state Z. The final state Z can be reached by either of the two paths shown in the figure. Which of the following choice(s) is(are) correct? (Take $$\Delta$$S as change in entropy and W as work done)
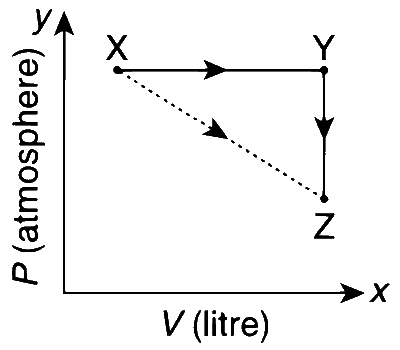
$$\Delta {S_{X \to Z}} = \Delta {S_{X \to Y}} + \Delta {S_{Y \to Z}}$$
$$\Delta {W_{X \to Z}} = \Delta {W_{X \to Y}} + \Delta {W_{Y \to Z}}$$
$${W_{X \to Y \to Z}} = {W_{X \to Y}}$$
$$\Delta {S_{X \to Y \to Z}} = \Delta {S_{X \to Y}}$$
Explanation
As entropy is a state function and is additive
$$\Delta {S_{X \to Z}} = \Delta {S_{X \to Y}} + \Delta {S_{Y \to Z}}$$
On moving from Y to Z, the work done is zero as the volume is kept constant (isochoric process), so
$${W_{X \to Y \to Z}} = {W_{X \to Y}}$$
Comments (0)


