JEE Advance - Chemistry (2012 - Paper 1 Offline - No. 16)
Identify the binary mixtures that can be separated into individual compounds, by differential extraction, as shown in the given scheme.
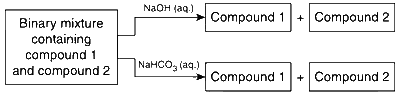
C6H5OH and C6H5COOH
C6H5COOH and C6H5CH2OH
C6H5CH2OH and C6H5OH
C6H5CH2OH and C6H5CH2COOH
Explanation
Both phenol and benzoic acid are soluble in NaOH, so they cannot be separated.
C6H5OH + NaOH $$\to$$ C6H5ONa + H2O
C6H5COOH + NaOH $$\to$$ C6H5COONa + H2O
Benzel alcohol C6H5CH2OH is not soluble in NaOH and NaHCO3, while benzoic acid is soluble in both, so these can be separated. Both C6H5OH and C6H5CH2OH are insoluble in NaHCO3, so they cannot be separated. C6H5CH2COOH is soluble in NaOH and NaHCO3, whereas benzyl alcohol is not soluble in NaOH and NaHCO3. So, they can be separated.
Comments (0)


