JEE Advance - Chemistry (2012 - Paper 1 Offline - No. 11)
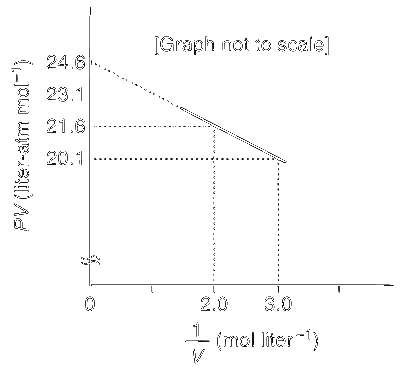
For one mole of a van der Waals gas when b = 0 and T = 300 K, the PV vs. 1/V plot is shown below. The value of the van der Waals constant a (atm L2 mol$$-$$2) is
1.0
4.5
1.5
3.0
Explanation
The van der Waals equation is
$$\left( {p + {a \over {{V^2}}}} \right)(V - b) = RT$$
but it is given that b = 0. So, the equation reduces to
$$\left( {p + {a \over {{V^2}}}} \right)V = RT \Rightarrow pV = - {a \over V} + RT$$
Comparing it with a straight line equation we get slope as $$-$$a. Calculating the slope, we get
$${{24.6 - 20.1} \over {3.0 - 0}} = 1.5 \Rightarrow a = 1.5$$
Comments (0)


