JEE Advance - Chemistry (2011 - Paper 2 Offline - No. 9)
The major product of the following reaction is
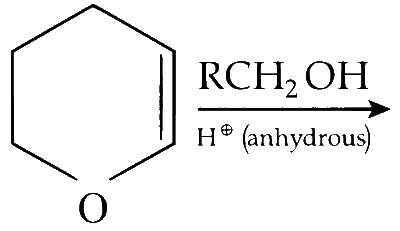
a hemiacetal
an acetal
an ether
an ester
Explanation
In presence of mineral acid $\mathrm{H}^{+}$(with no water) positively charged centre is generated at second position on the ring.
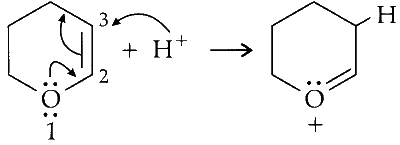
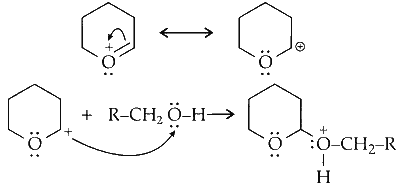
Since, oxygen is electronegative, it shifts pi electron towards itself generating a carbocation which attack one lone pair of electron on oxygen of alcohol.
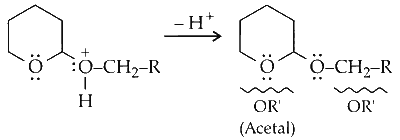
Loss of proton $\left(\mathrm{H}^{+}\right)$from oxygen generates acetal.
Comments (0)


