JEE Advance - Chemistry (2011 - Paper 2 Offline - No. 8)
Among the following complexes (K-P),
K3[Fe(CN)6] (K), [Co(NH3)6]Cl3 (L), Na3[Co(oxalate)3] (M), [Ni(H2O)3]Cl2 (N), K2[Pt(CN)4] (O) and [Zn(H2O)6(NO3)2] (P)
The diamagnetic complexes are
Explanation
In K3Fe(CN)6 :
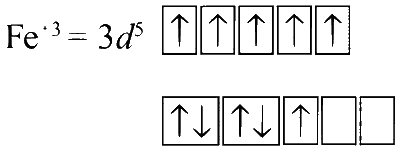
CN$$-$$ being a strong field ligand, it causes pairing of electrons, so only one unpaired electron. The complex is paramagnetic.
In Co[(NH3)6]Cl3 :
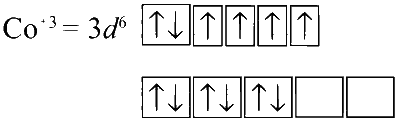
NH3 being a strong field ligand it causes pairing of spins. There is no unpaired electron, hence the complex is diamagnetic.
In Na3[Co(oxalate)3] :
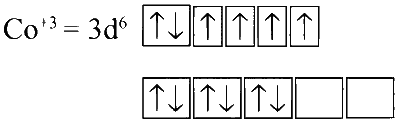
Oxalate being a strong field ligand it causes pairing of electron spins. There is no unpaired electron, hence the complex is diamagnetic.
In [Ni(H2O)6]Cl2 :
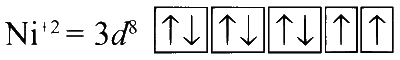
Since H2O is a weak field ligand, no pairing takes place. There are two unpaired electrons, so the complex is paramagnetic.
In K2[Pt(CN)4] :
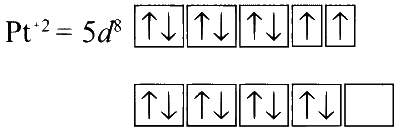
CN$$-$$ being a strong field ligand, it causes pairing of electrons spins, so no unpaired electron. Hence, the complex is diamagnetic.
In [Zn(H2O)6(NO3)2] : Zn2+ has 3d10 configuration. Therefore, it is a diamagnetic complex.
Comments (0)


