JEE Advance - Chemistry (2011 - Paper 2 Offline - No. 14)
The correct functional group X and the reagent/reaction condition Y in the following scheme are
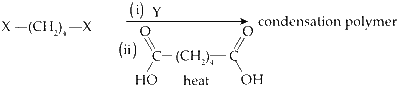
Explanation
(i) (a) Hexa-1, 6-diamide undergoes Hoffmann bromamide degradation to form tetra-1, 4-diammine.
If $$ \mathrm{X}=-\mathrm{CONH}_2 $$
$$ \mathrm{H}_2 \mathrm{NOC}-\left(\mathrm{CH}_2\right)_4-\mathrm{CONH}_2+2 \mathrm{Br}_2+4 \mathrm{NaOH}(a q) \rightarrow $$
$\mathrm{H}_2 \mathrm{~N}-\left(\mathrm{CH}_2\right)_4-\mathrm{NH}_2+2 \mathrm{Na}_2 \mathrm{CO}_3+4 \mathrm{NaBr}(a q)+4 \mathrm{H}_2 \mathrm{O}$
(b) Hexa-1, 6-diammine reacts with hexa-1, 6-dioic acid to form an amide with release water.
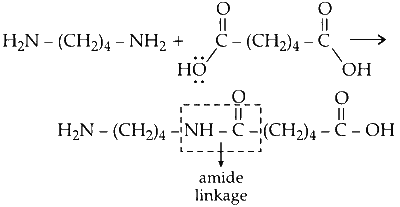
Since, water is lost during the reaction between amine and carboxylic acid, the reaction is condensation reaction.
Option (C) is correct.
(ii) (a) Hexa-1, 6-dinitrile undergoes reduction to amine in pressure of same hydrogen and nickel as catalyst to form hexane-1, 6-diammine. If $X$ $=\mathrm{CN}$
$$ \mathrm{NC}-\left(\mathrm{CH}_2\right)_4-\mathrm{CN} \xrightarrow[\mathrm{Ni}(\mathrm{~s})]{\mathrm{H}_2(\mathrm{~g})} \quad \mathrm{H}_2 \mathrm{~N}-\left(\mathrm{CH}_2\right)_6-\mathrm{NH}_2\text { (hexane-1, 6-diammine) } $$
(b) Hexane-1, 6-diammine reacts with hexane-1, 4-dioic acid to form an amide with the loss of water molecule. This reaction is condensation reaction.
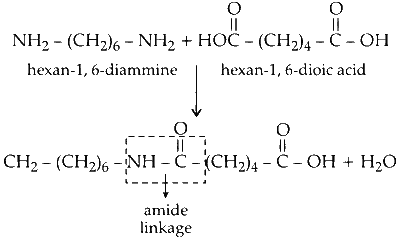
Since, water molecule is lost during the reaction between amine and carboxylic acid the reaction is condensation reaction.
Option (D) is correct.
(iii) The compound contains carbonyl functional group of ester and cannot be reduced by hydrogen in presence of nickel. Hence, no condensation reaction is possible between ester and an acid.
If $\mathrm{X}=\mathrm{COOCH}_3$
$$ \mathrm{H}_3 \mathrm{COOC}-\left(\mathrm{CH}_2\right)_4-\mathrm{COOCH}_3+\mathrm{H}_{2(\mathrm{~g})} \xrightarrow{\mathrm{Ni}(\mathrm{~s})} $$No reaction
$$ \mathrm{H}_3 \mathrm{COOC}-\left(\mathrm{CH}_2\right)_4-\mathrm{COOCH}_3\text { (hexan-1, 4-dioic acid) }+\mathrm{HOOC}-\left(\mathrm{CH}_2\right)_4 $$
Option (A) is not correct.
(iv) Hexan-1, 4-diamide is not affected by the reducing agent hydrogen in presence of catalyst nickel. Reaction between the hexan-1, 4-diamine with basic functional group $\left(-\mathrm{NH}_2\right)$ and acidic proton of hexane-1, 4-dioic acid gives salt.
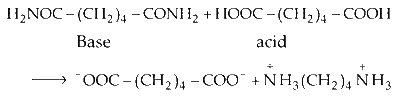
Option (B) is correct.
Comments (0)


