JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 5)
Explanation
(i) The structures of the different organic compounds are as follows :
(A) p-Nitrophenol
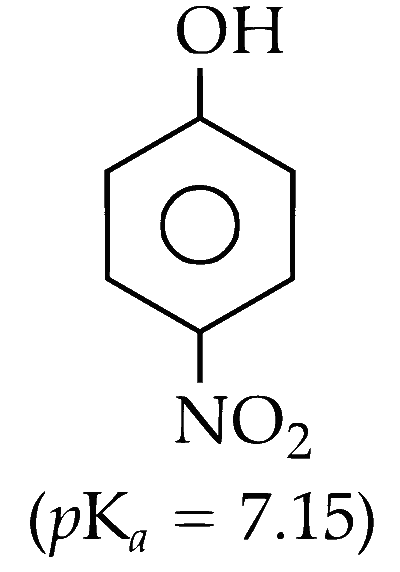
(B) p-Hydroxybenzoic Acid
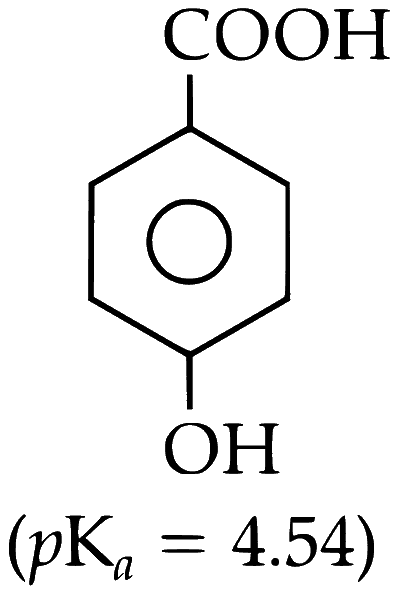
(C) o-Hydroxybenzoic Acid
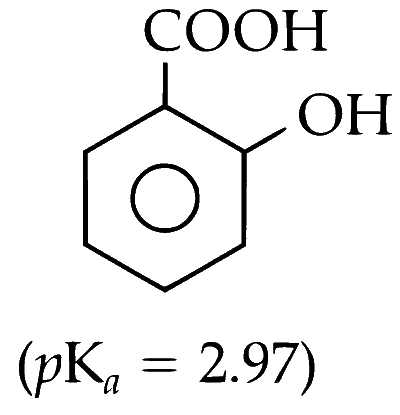
(D) p-Toluic Acid
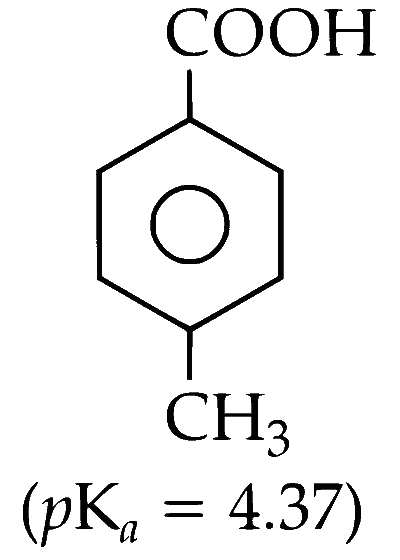
(ii) According to the $pK_a$ values, the order of acidity of these compounds is as follows :
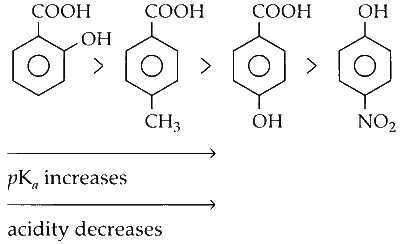
(iii) Regardless of the substitution, phenols are generally less acidic than carboxylic acids. Therefore, p-nitrophenol is the least acidic, despite the presence of an electron-withdrawing nitro group.
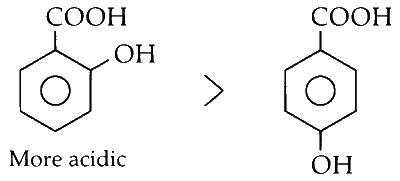
(iv) The "ortho effect," i.e., the presence of an electron-withdrawing or releasing substituent at the ortho position relative to the carboxylic acid group, increases the acidity of substituted benzoic acids.
Therefore, o-hydroxybenzoic acid is more acidic than p-hydroxybenzoic acid.
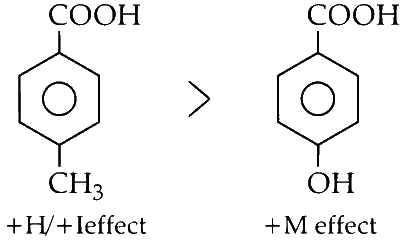
(v) The presence of an -OH group at the para position decreases the acidity of carboxylic acids due to its electron-donating nature (via the positive mesomeric effect, +M). This effect is stronger compared to the electron-donating nature of a methyl group via the positive inductive effect (+I) or hyperconjugative effect (+H).
Hence, the order of acidity of the compounds is :
(C) o-Hydroxybenzoic Acid
(D) p-Toluic Acid
(B) p-Hydroxybenzoic Acid
(A) p-Nitrophenol
Option (C) has the lowest $pK_a$ or the highest $K_a$.
Comments (0)


