JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 3)
Explanation
(A) When gas molecules collide with each other, high energy molecule transfers some of its energy to less energetic molecule, but the total energy remains conserved. This type of collision between gas molecules is called elastic collision. According to kinetic theory of gas, no loss of energy is observed on collisions and kinetic energy is proportional to absolute temperature of gas.
Option (A) is correct.
(B) Momentum of any particle for example, gas molecules depends upon the mass as well as the velocity of the gas. But when there is huge increase in the mass of the gas, its velocity decreases considerably. Hence, heavier gas molecules collides the walls of container with less impact. The transfer of momentum to the walls is very less.
Option (B) is incorrect.
(C) Maximum number (no.) of gas molecules have speed distributed around the most probable speed ( $v_{\mathrm{mps}}$ ). This speed corresponds to the maximum peak of Maxwell-Boltzmann distribution curve.
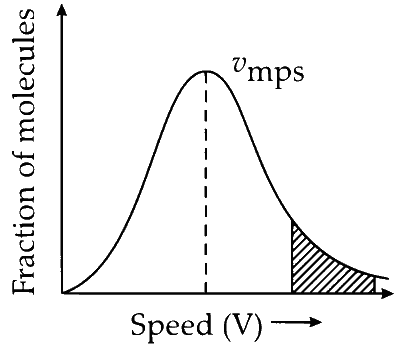
There are few molecules which have very high speed (the shaded area under the curve).
Option $(\mathrm{C})$ is correct.
Option (D) is correct.
Comments (0)


