JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 22)
The total number of alkenes possible by dehydrobromination of 3-bromo-3-cyclopentylhexane using alcoholic KOH is _______.
Answer
5
Explanation
(1) The structure of 3-bromo-3-cyclopentylhexane -
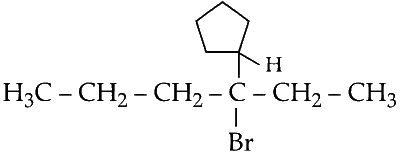
(2) Since, bromide (Br–) is a good leaving group, elimination using strong base takes place using strong base (KOH) take via E2 mechanism. This is also called $$\beta $$ elimination.
(3) There are 3 different types of protons :
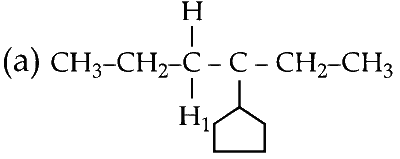
(iv) The strong base abstracts $$\beta $$ hydrogen (H1 or H2 or H3) with simultaneous loss of bromide ion forming an alkene. This alkene can exist in 2 conformations, E and Z.
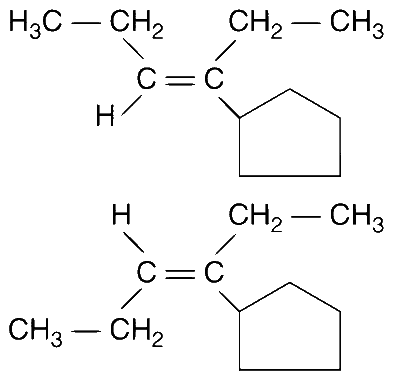
(a) Elimination of H1 proton :
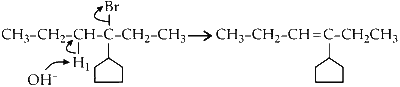
This product can exist in 2 conformations, E and Z.
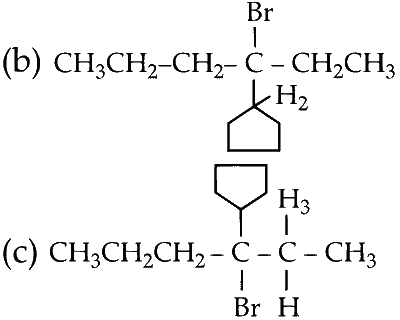
(b) Elimination of H2 proton :
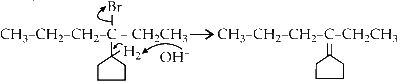
Since, the groups about sp2 hybridised carbon of cyclopentane is fixed no E and Z forms are possible. This molecule has only one conformation.
(c) Elimination of H3 proton :
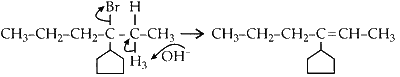
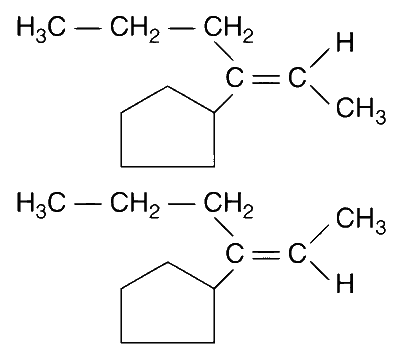
This product can exist in 2 conformations, E and Z.
Hence, a total of 5 products are possible.

(2) Since, bromide (Br–) is a good leaving group, elimination using strong base takes place using strong base (KOH) take via E2 mechanism. This is also called $$\beta $$ elimination.
(3) There are 3 different types of protons :


(iv) The strong base abstracts $$\beta $$ hydrogen (H1 or H2 or H3) with simultaneous loss of bromide ion forming an alkene. This alkene can exist in 2 conformations, E and Z.
(a) Elimination of H1 proton :
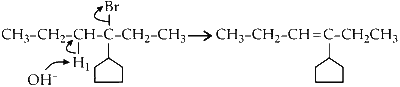
This product can exist in 2 conformations, E and Z.

(b) Elimination of H2 proton :
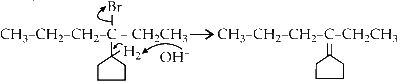
Since, the groups about sp2 hybridised carbon of cyclopentane is fixed no E and Z forms are possible. This molecule has only one conformation.
(c) Elimination of H3 proton :
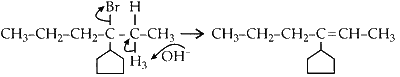
This product can exist in 2 conformations, E and Z.

Hence, a total of 5 products are possible.
Comments (0)


