JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 2)
Explanation
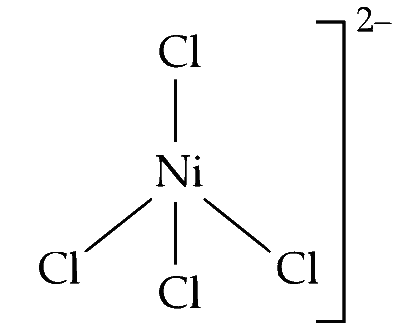
(i) Nickel in +2 oxidation state $\left(\mathrm{Ni}^{2+}\right)$ prefers to form tetrahedral complexes with chlorine, e.g., $\left[\mathrm{NiCl}_4\right]^{2-}$.
The oxidation state of nickel in $\left[\mathrm{NiCl}_4\right]^{2-}$
$$ \begin{gathered} x+4 \times(-1)=-2 \\\\ x=-2+4=+2 \end{gathered} $$
Electronic configuration of $\mathrm{Ni}^{2+}=[\mathrm{Ar}] 3 d^8 4 s^0$
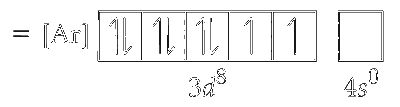
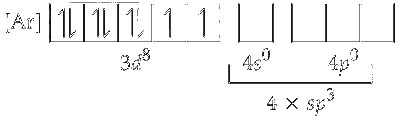
Since, chlorine is a weak ligand, electrons in $\mathrm{Ni}^{2+}$ do not pair up. One $4 s$ and three $4 p$ orbitals undergo hybridisation to form four $s p^3$ hybrid orbitals. These vacant orbitals accept a pair of electron from each of the chloride ion.
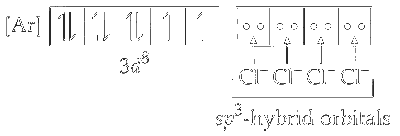
The geometry of $s p^3$ hybridised nickel $\left[\mathrm{Ni}^{2+}\right]$ is tetrahedral
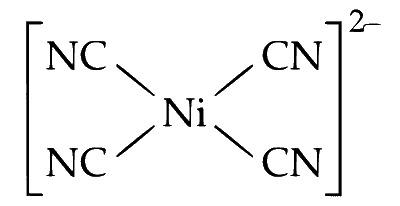
(ii) Nickel in +2 oxidation state $\left(\right.$ as $\left.\mathrm{Ni}^{2+}\right)$ forms square planner complex with cyanide ligands, e.g., $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$.
Oxidation state of $\mathrm{Ni}$ in $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$
$$ \begin{aligned} x+4 \times(-1) & =-2 \\ x & =-2+4=+2 \end{aligned} $$
The oxidation state of nickel in $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ is $+2$
Electronic configuration of $\mathrm{Ni}^{2+}=[\operatorname{Ar}] 3 d^8 4 s^0$
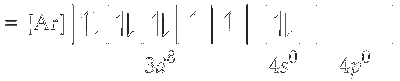
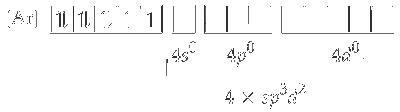
Since, cyanide is a strong ligand, electrons in $3 d$-orbitals are paired up.
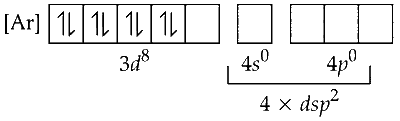
One $3 d$, one $4 s$ and two $4 p$ orbitals undergoes hybridisation to form four $d s p^2$ hybrid orbitals. These vacant orbitals accept a pair of electrons from each of cyanide ligand.
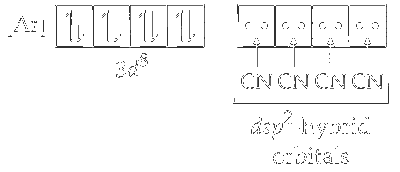
The geometry of $d s p^2$ hybridised nickel complex $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ is square planner.
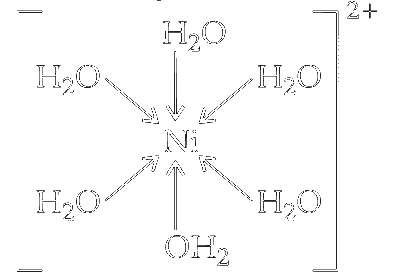
(iii) Nickel in +2 oxidation state as $\mathrm{Ni}^{2+}$ forms octahedral complex with water as ligands, e.g., $\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$
Oxidation state of nickel in $\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ is:
$$ \begin{aligned} x+6 \times 0 & =+2 \\\\ x & =+2 \end{aligned} $$
Electronic configuration of $\mathrm{Ni}^{2+}=$
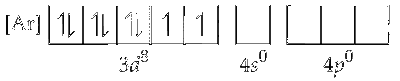
Since, water is a weak ligand, electrons in $3 d$ orbitals of Nickel remains unpaired. One $4 s$, three $4 p$ and two $4 d$ orbitals undergo hybridisation to form $s p^3 d^2$ hybrid orbitals. These vacant orbitals accept lone pair of electron from each of the water molecule.
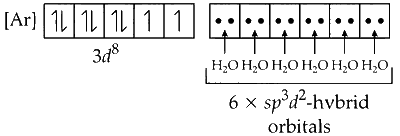
The geometry of $s p^3 d^2$ hybridised nickel complex $\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}$ is octahedral.
The geometries of different $\mathrm{Ni}^{2+}$ complexes are as follows:
$$ \begin{aligned} {\left[\mathrm{NiCl}_4\right]^{2-} } & \rightarrow \text { tetrahedral } \\\\ {\left[\mathrm{NiCN}_4\right]^{2-} } & \rightarrow \text { square planner } \\\\ {\left[\mathrm{Ni}_{(}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+} } & \rightarrow \text { octahedral } \end{aligned} $$
Comments (0)


