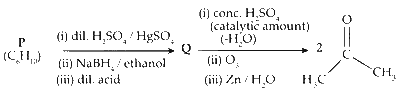JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 16)
The structure of compound P is
CH3CH2CH2CH2 $$-$$ C $$\equiv$$ C $$-$$ H
H3CH2C $$-$$ C $$\equiv$$ C $$-$$ CH2CH3
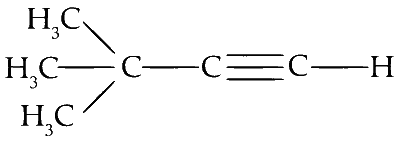
Explanation
(i) When 3,3-dimethyl but-1-yne reacts with dil. $\mathrm{H}_2 \mathrm{SO}_4$ in presence of $\mathrm{HgSO}_4$, ketone is formed.
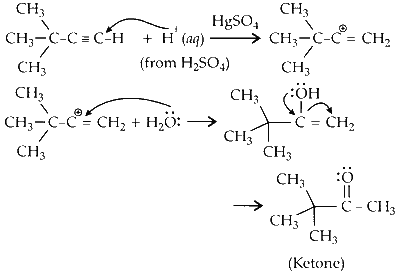
(ii) The ketone is reduced to secondary alcohol in presence of sodium borohydride $\left(\mathrm{NaBH}_4\right)$ ethanol).
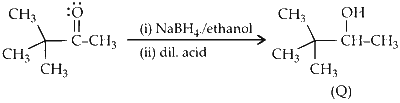
(iii) Reaction of acid with alcohol results in dehydration forming an alkene. During the process, methyl shift results in rearrangement of carbocation which further leads to the formation of alkene.
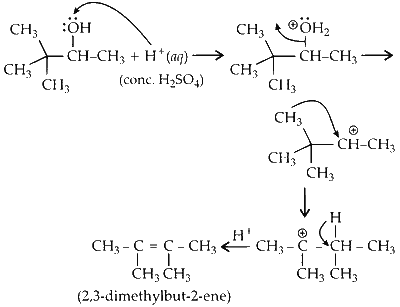
The product 2,3-dimethyl but-2-ene undergoes ozonolysis to form two moles acetone.
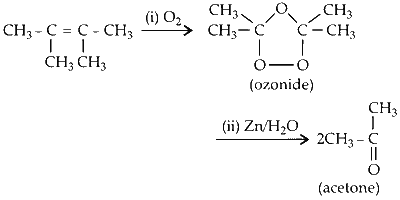
Two moles of acetone is the final product of the reaction.
Compound P is 3,3-dimethyl butyne.
Comments (0)