JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 15)
Among the given options, the compound(s) in which all the atoms are in one plane in all the possible conformations (if any) is(are)
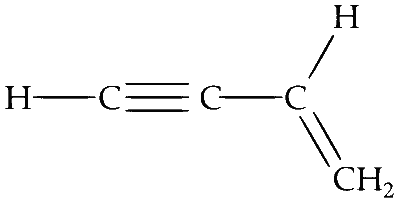
H2C = C = 0
H2C = C = CH2
Explanation
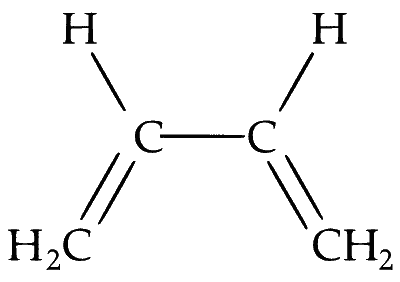
For compound in option (A) : Only two of the conformers (cisiod and transoid) have all the atom in the same plane.
For compound in option (B) : The terminal hydrogen of allene will be perpendicular to each other plane.
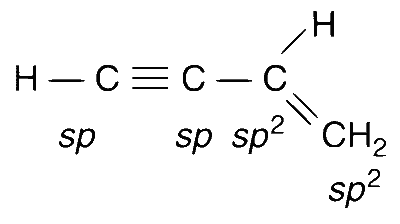
For compound in option (C) : All the atoms are in one plane in all the possible conformations. There is no atom on oxygen.
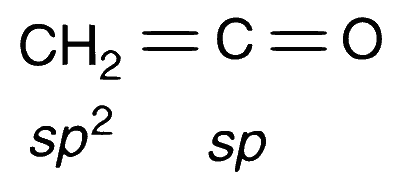
Comments (0)


