JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 13)
The major product of the following reaction is
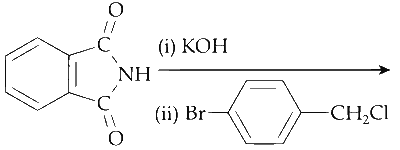
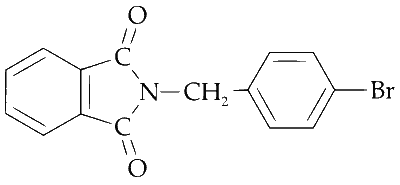
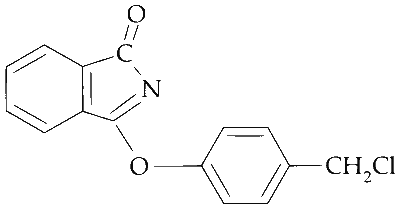
Explanation
The reactant, phthalimide, undergoes an acid-base reaction with KOH. In this reaction, the proton from the nitrogen reacts with the OH- from the base to form water.
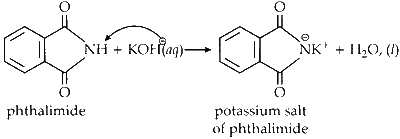
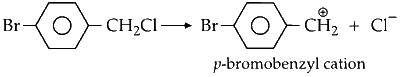
The resulting salt then undergoes a substitution reaction with p-bromobenzyl chloride to form the major product. This reaction proceeds via an SN1 mechanism due to the stability of the p-bromobenzyl cation.
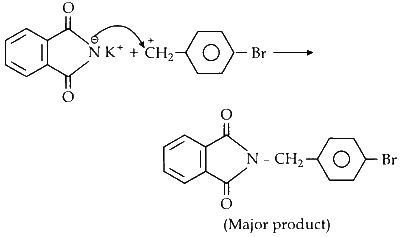
Comments (0)


