JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 12)
AgNO3(aq.) was added to an aqueous KCl solution gradually and the conductivity of the solution was measured. The plot of conductance ($$\Lambda $$) versus the volume of AgNO3 is
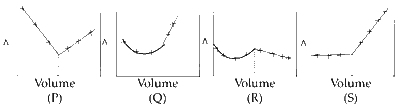
Explanation
The plot obtained from adding AgNO3(aq.) to a solution of KCl is as follows :
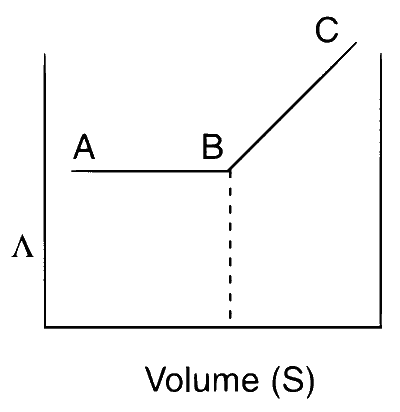
The reaction occurring is :
$$ Ag^ + + NO_3^- + K^ + + Cl^- \to AgCl + K^ + + NO_3^- $$
Or more succinctly :
$$ AgNO_3(aq.) + K^+Cl^-(aq.) \to AgCl(s) + KNO_3 $$
Upon the gradual addition of aqueous AgNO3, precipitation does not begin immediately. The precipitation of AgCl starts only when the ionic product of AgCl exceeds its solubility product. During this initial phase, AgNO3 precipitates as AgCl, simultaneously adding NO3- ions to the solution. Since the total number of ions remains constant, the conductance does not change, represented by the flat segment AB in the figure. Once precipitation is complete, any further addition of AgNO3 increases the ion concentration in the solution, thus increasing the conductance, shown by the rising segment BC.
Comments (0)


