JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 11)
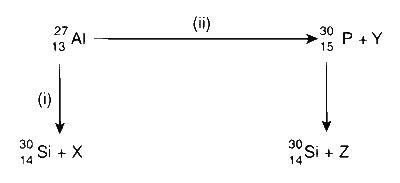
Bombardment of aluminium by $$\alpha$$-particle leads to its artificial disintegration in two ways : (i) and (ii) as shown. Products X, Y and Z, respectively, are
Explanation
Bombardment of aluminum by an $$\alpha$$-particle leads to its artificial disintegration in two ways, as shown in the reactions below. The resultant products X, Y, and Z are identified as follows :
Reaction (i)
Disintegration of aluminum into silicon :
$$^{4}_{2} \text{He} + ^{27}_{13} \text{Al} \rightarrow ^{30}_{14} \text{Si} + ^{A}_{Z} X$$
Conservation conditions :
(a) Charge balance :
$ 2 + 13 = 14 + Z \implies Z = 1 $
(b) Mass balance :
$ 4 + 27 = 30 + A \implies A = 1 $
The particle $A_Z X$ is $^1_1 \text{H}$, a proton (an isotope of hydrogen).
Reaction (ii)
Disintegration of aluminum into phosphorus :
$$^{4}_{2} \text{He} + ^{27}_{13} \text{Al} \rightarrow ^{30}_{15} \text{P} + ^{A}_{Z}Y$$
Conservation conditions :
(a) Charge balance :
$ 2 + 13 = 15 + Z \implies Z = 0 $
(b) Mass balance :
$ 4 + 27 = 30 + A \implies A = 1 $
The particle $Y$ has one unit mass and no charge, which is a neutron $^1_0 n$.
Reaction (iii)
Disintegration of phosphorus into silicon :
$$^{30}_{15}\text{P} \longrightarrow \, ^{30}_{14}\text{Si} + ^{A}_{Z}\text{Z} $$
Conservation conditions :
(a) Mass balance :
$ 30 = 30 + A \implies A = 0 $
(b) Charge balance :
$ 15 = 14 + Z \implies Z = 1 $
The particle has zero mass but one unit of positive charge. Hence, the particle is a positron $\left(^{0}_{+1}\beta\right)$.
Summary
The particles are :
X = $^1_1 \text{H}$ (proton)
Y = $^1_0 n$ (neutron)
Z = $^{0}_{+1}\beta$ (positron)
Comments (0)


