JEE Advance - Chemistry (2011 - Paper 1 Offline - No. 10)
The difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is
Answer
5
Explanation
(i) The structure of compound containing sulphur in $\mathrm{Na}_2 \mathrm{~S}_4 \mathrm{O}_6$ is :
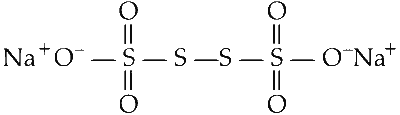
Let the oxidation state of sulpher be $x$ :
$$ \begin{gathered} 4 \times x+2 \times(+1)+6 \times(-2)=0 \\ 4 x=12-2=0 \\ 4 x=10 \\ x=\frac{5}{2}=2.5 \end{gathered} $$
(ii) Each of the corner sulphurs utilises five valence electrons to form bond with oxygen atoms.
Their oxidation state is +5.
(iii) Oxidation state of central sulphur atom is zero (0).
Difference between two types of sulphur = 5 – 0 = 5.
Comments (0)


