JEE Advance - Chemistry (2010 - Paper 2 Offline - No. 9)
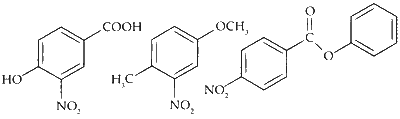
The compounds $\mathbf{P}, \mathbf{Q}$ and $\mathbf{S}$ were separately subjected to nitration using $\mathrm{HNO}_3 / \mathrm{H}_2 \mathrm{SO}_4$ mixture. The major product formed in each case respectively, is :
Explanation
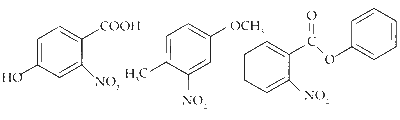
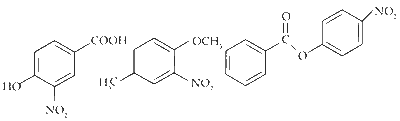
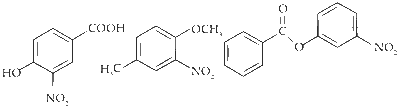
$$ \text { The products obtained on nitration of } \mathbf{P}, \mathbf{Q} \text { and } \mathbf{S} \text { are } $$
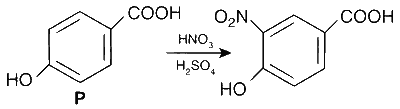
Here strongly activating and ortho-, para directing -OH group determines the site for electrophilic substitution.
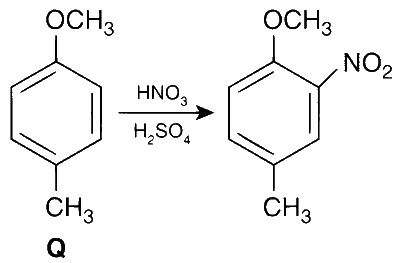
Here $-\mathrm{OCH}_3$ is a stronger activator than $-\mathrm{CH}_3$ and both are ortho-, para directing.
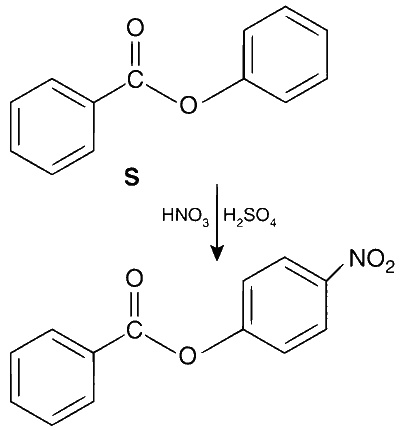
Here, the substitution takes place on the activated ring (with substituent $\mathrm{PhCOO}^-$ ) at the sterically unhindered para-position.
Comments (0)


