JEE Advance - Chemistry (2010 - Paper 2 Offline - No. 8)
The total number of diprotic acids among the following is:
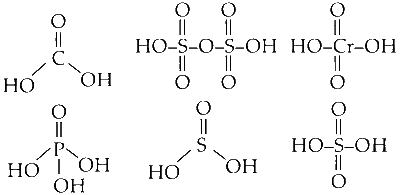
H3PO4, H2SO4, H3PO3, H2CO3, H2S2O7, H3BO3, H3PO2, H2CrO4 and H2SO3
Answer
6
Explanation
A diprotic acid is an acid that contains within its molecular structure two hydrogen atoms per molecule capable of dissociating (i.e., ionisable protons) in water.
$$ \mathrm{H}_2 \mathrm{SO}_4, \mathrm{H}_2 \mathrm{CO}_3, \mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_7, \mathrm{H}_2 \mathrm{CrO}_4, \mathrm{H}_3 \mathrm{PO}_3, \mathrm{H}_2 \mathrm{SO}_3 $$
Structure of each of the compounds is given below :
Comments (0)


