JEE Advance - Chemistry (2010 - Paper 2 Offline - No. 18)
| Column I | Column II |
|---|---|
(A) 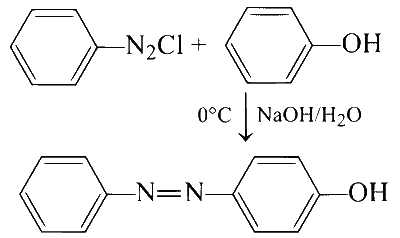
|
(P) Racemic mixture |
(B) 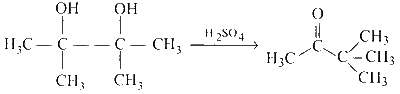
|
(Q) Addition reaction |
(C) 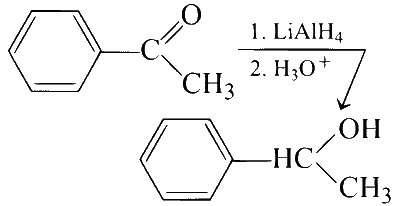
|
(R) Substitution reaction |
(D) 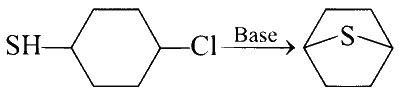
|
(S) Coupling reaction |
Explanation
(i) It is an example of electrophilic substitution reaction which results in the formation of a coupled product. The nucleophilic nitrogen attacks at electron rich carbon of phenol at para position as follows:
$$ A \rightarrow R \text { and } S $$
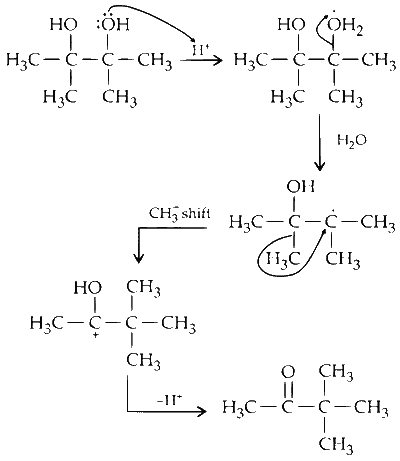
(ii) The reaction represents Pinacole-pinacolone rearrangement. In this reaction, the intermediate is carbocation.
The reaction is represented as follows:
$$ B \rightarrow T $$
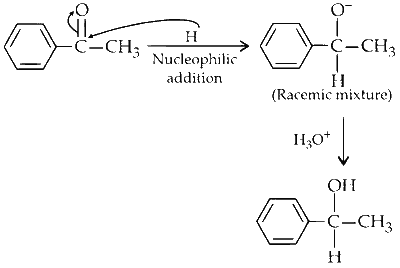
(iii) It is an example of addition reaction to carbonyl group, where lithium aluminium hydride $\left(\mathrm{LiAlH}_4\right)$ adds hydrogen across carbon and oxygen of carbonyl group thus reducing it to an alcohol. Both R and S enantiomers will be formed. Hence, racemic mixture will be obtained as carbonyl carbon becomes chiral.
(C) $$ \to $$ (P, Q)
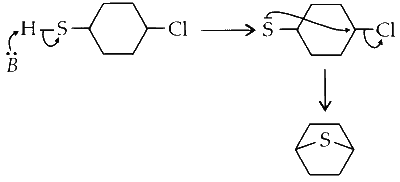
(iv) It is an example of nucleophilic substitution, where electron rich sulphur (due to lone pairs) displaces chlorine on the ring forming a bicyclic compound.
$$ D \rightarrow R $$
Comments (0)


