JEE Advance - Chemistry (2010 - Paper 2 Offline - No. 11)
The complex showing a spin-only magnetic moment of 2.82 B.M. is :
$\mathrm{Ni}(\mathrm{CO})_4$
$\left[\mathrm{NiCl}_4\right]^{2-}$
$\mathrm{Ni}\left(\mathrm{PPh}_3\right)_4$
$\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$
Explanation
$\begin{aligned} & {\left[\mathrm{NiCl}_4\right]^{2-}} \\\\ & \text { Oxidation state of }\left[\mathrm{NiCl}_4\right]^{2-}=+2 \\\\ & \mathrm{Ni}(28)=[\mathrm{Ar}] 3 d^8 4 s^2 \\\\ & \mathrm{Ni}^{2+}=[\mathrm{Ar}] 3 d^8\end{aligned}$
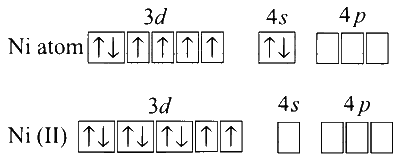
Among the given complexes of Ni , only $\mathrm{Cl}^{-}$is a weak ligand and does not cause pairing of electrons to take place.
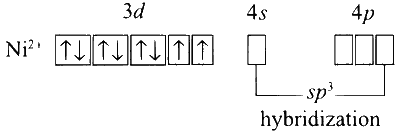
Thus, the spin only magnetic moment, with two unpaired electrons $(n=2)$ configuration is
$$ \sqrt{n(n+2)}=\sqrt{2(2+2)}=2.82 \mathrm{BM}=2.82 \mathrm{BM} $$
Comments (0)


