JEE Advance - Chemistry (2010 - Paper 2 Offline - No. 1)
The species having pyramidal shape is
SO3
BrF3
$$SiO_3^{2-}$$
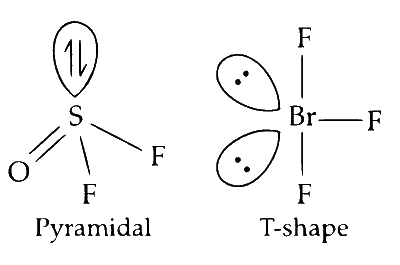
OSF2
Explanation
$\mathrm{OSF}_2$ has $s p^3$ hybridisation. According to VSEPR theory, we can conclude that the shape of $\mathrm{OSF}_2$ is a trigonal pyramid.
Sulphur has a non-bonding pair of electrons and three sulphur-oxygen ( $\mathrm{S}-\mathrm{O})$ bond pairs. $\mathrm{SO}_2$ is $s p^2$ hybridised with three bond pairs (two sigma and one pi) and one lone pair. It is replace trigonal by bent.
$\mathrm{BrF}_3$ is $s p^3 d$ hybridized with three bond pair and two lone pairs. It is T shaped.
$\mathrm{SiO}_3^{2-}$ is $s p^2$ hybridised with four bond pairs (three sigma and one pi) and no lone pair. It has trigonal planar shape.
Comments (0)


