JEE Advance - Chemistry (2010 - Paper 1 Offline - No. 8)
Explanation
To get appropriate bromoalkane an alkyne that were involved in the synthesis, we have to cleave the molecule and we know that sodium amide is involved, so the synthesis of 3-octyne using the alkyne and bromoalkane was $\mathrm{S}_{\mathrm{N}}2$ reaction. And as the sodium amide is involved, the alkyne involved must be terminal then only it could abstract the acidic hydrogen 3-octyne is a molecule that will have eight carbon atoms in their parent chain since the prefix-oct represents 8 and as in the name it is given that 3 octyne, ' 3 ' represents the position of triple bond. Thus suffix-one represents triple bond.
So, in the molecule triple bond will be at the third carbon. Structure of 3-octyne :
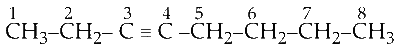
Now, cleave the molecule between $\mathrm{C}_2-\mathrm{C}_3$ or $\mathrm{C}_4$ - $C_5$.
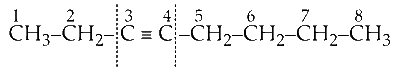
Now, see the products formed if cleavage takes place between C2-C3, the reactants will be
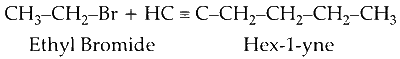
If cleavage takes place between C4-C5, the reactants will be :
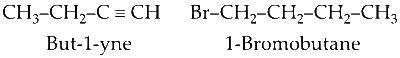
Then correct option is D.
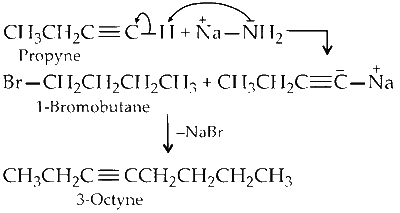
Comments (0)


