JEE Advance - Chemistry (2010 - Paper 1 Offline - No. 21)
In the reaction
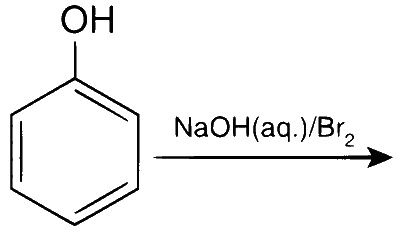
The intermediate(s) is(are)
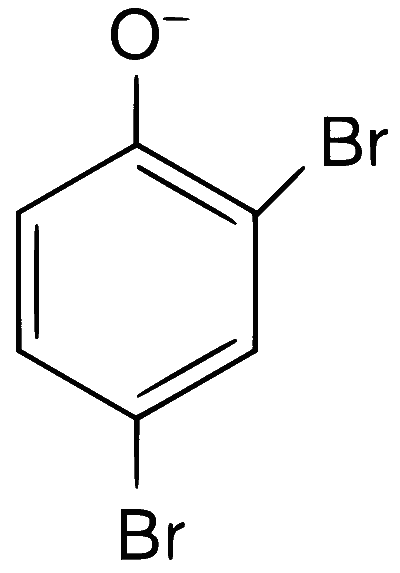
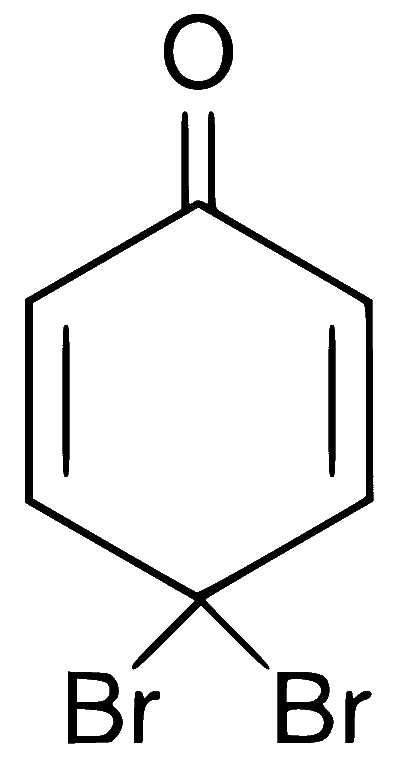
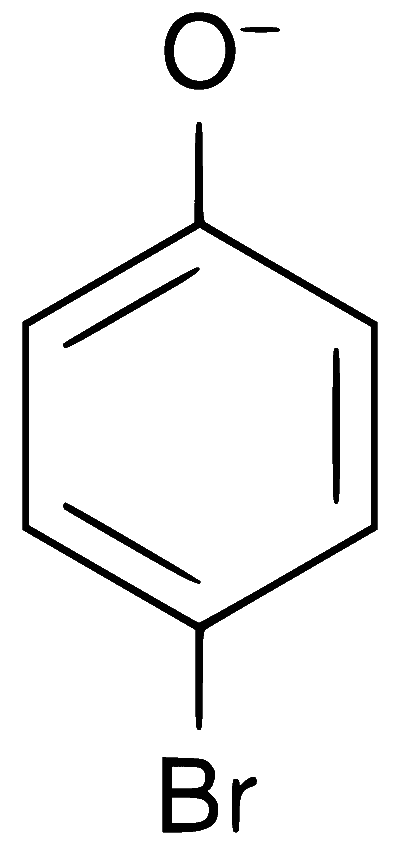
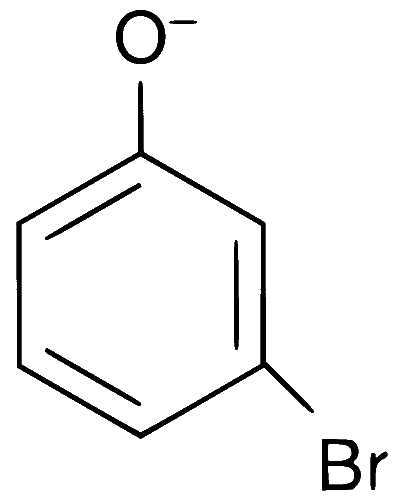
Explanation
In presence of base, phenol forms phenoxide ion which is $$ortho$$-$$para$$ directing because in the resonating structures, the electron density increases at ortho and para positions. The phenoxide ion further undergoes electrophilic substitution with Br$$_2$$ at $$ortho$$ and $$para$$ positions.
Comments (0)


