JEE Advance - Chemistry (2010 - Paper 1 Offline - No. 17)
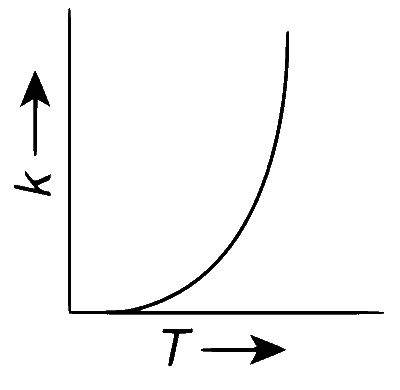
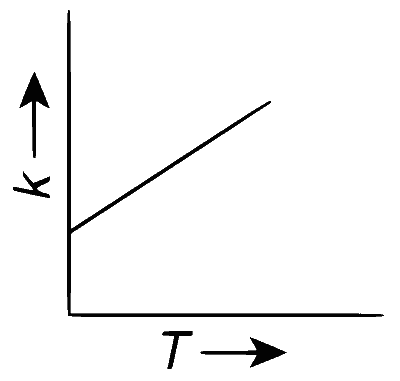
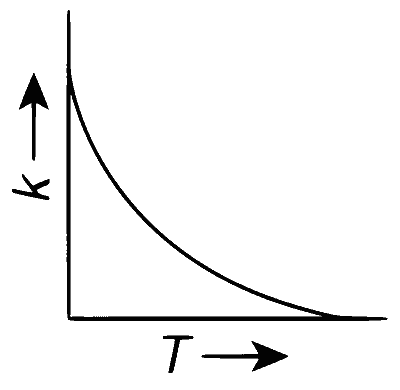
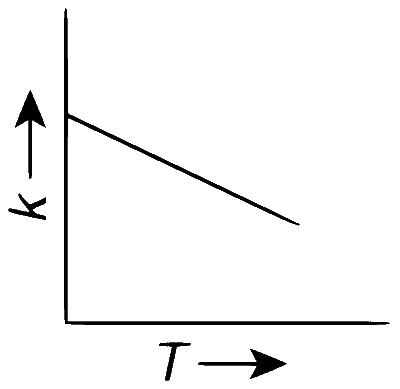
Plots showing the variation of the rate constant ($$k$$) with temperature ($$T$$) are given below. The point that follows Arrhenius equation is
Explanation
According to the Arrhenius equation
$$k = A{e^{ - {E_a}/RT}}$$
where, k = rate constant, Ea = activation energy and T = temperature
From the expression, we have that as temperature increases, the rate constant ($$k$$) increases exponentially.
Comments (0)


