JEE Advance - Chemistry (2010 - Paper 1 Offline - No. 1)
Based on VSEPR theory, the number of 90 degree F-Br-F angles in BrF5 is
Answer
0
Explanation
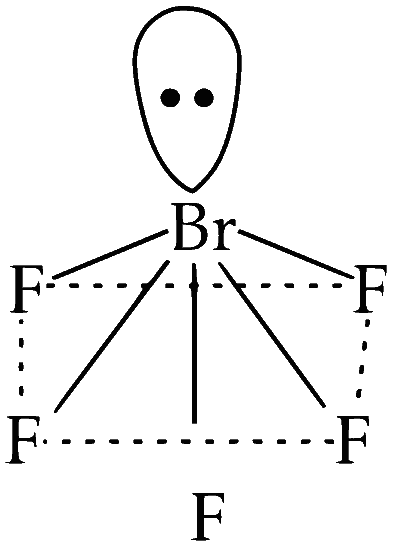
According to VSEPR, $\mathrm{BrF}_5$ has square pyramidal structure with axial plane containing a lone pair and fluorine. The other four fluorine are arranged in square planner configuration around central metal atom. Thus, $\mathrm{BrF}_5$ assumes square pyramidal shape where the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimises this repulsion, thus, determining the molecule's geometry. All four planar bonds $(\mathrm{F}-\mathrm{Br}-\mathrm{F})$ will reduce from $90^{\circ}$ to $84.8^{\circ}$ after lone pair - bond pair repulsion.
So, there are no 90 -degree $\mathrm{F}-\mathrm{Br}-\mathrm{F}$ angles in $\mathrm{BrF}_5$.
Comments (0)


