JEE Advance - Chemistry (2009 - Paper 1 Offline - No. 20)
Match each of the compounds in Column I with its characteristic reaction(s) in Column II.
| Column I | Column II | ||
|---|---|---|---|
| (A) | $$C{H_3}C{H_2}C{H_2}CN$$ | (P) | Reduction with $$Pd - C/{H_2}$$ |
| (B) | $$C{H_3}C{H_2}OCOC{H_3}$$ | (Q) | Reduction with $$SnC{l_2}/HCl$$ |
| (C) | $$C{H_3} - CH = CH - C{H_2}OH$$ | (R) | Development of foul smell on treatment with chloroform and alcoholic KOH |
| (D) | $$C{H_3}C{H_2}C{H_2}C{H_2}N{H_2}$$ | (S) | Reduction with diisobutylaluminium hydride (DIBAL-H) |
| (T) | Alkaline hydrolysis |
$$\mathrm{(A)\to(P),(Q),(S),(T);(B)\to(Q),(T);(C)\to(P);(D)\to(S)}$$
$$\mathrm{(A)\to(Q), (R), (S),(T);(B)\to(S),(T);(C)\to(P);(D)\to(R)}$$
$$\mathrm{(A)\to(P),(R),(S),(T);(B)\to(S),(T);(C)\to(P);(D)\to(R), (T)}$$
$$\mathrm{(A)\to(P),(Q),(S),(T);(B)\to(S),(T);(C)\to(P);(D)\to(R)}$$
Explanation
(A) 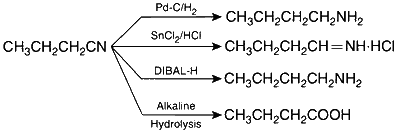
(B) Reaction with DIBAL-H and alkaline hydrolysis:
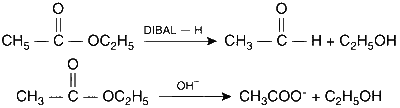
(C) Reduction with Pd-C/H$$_2$$:
$$C{H_3} - CH = CH - C{H_2}OH\buildrel {Pd - C/C{H_2}} \over \longrightarrow C{H_3} - C{H_2} - C{H_2} - C{H_2}OH$$
(D) Foul smell on treatment with $$CHC{l_3} + KOH$$:
$$RN{H_2}\buildrel {CHC{l_3} + KOH} \over \longrightarrow \mathop {RNC}\limits_{Foul\,smell} + KCl + {H_2}O$$
Comments (0)


