JEE Advance - Chemistry (2009 - Paper 1 Offline - No. 15)
The compound Z is
$$M{g_2}[Fe{(CN)_6}]$$
$$Fe[Fe{(CN)_6}]$$
$$F{e_4}{[Fe{(CN)_6}]_3}$$
$${K_2}Z{n_3}{[Fe{(CN)_6}]_2}$$
Explanation
The compound X is H$$_2$$S:
$$\mathrm{\mathop {N{a_2}S}\limits_{(X)} + 2{H^ + } \to {H_2}S + 2N{a^ + }}$$
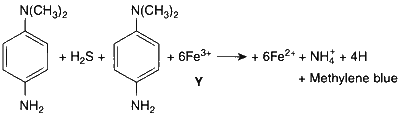
Thus, the compound Y is FeCl$$_3$$.
Compound Y on reaction with potassium hyxacyanoferrate(II) forms intense blue precipitate which dissolves on addition of reagent
$$\mathrm{4FeC{l_3} + 3{K_4}\left[ {Fe(II){{(CN)}_6}} \right] \to \mathop {F{e_4}{{[Fe{{(CN)}_6}]}_3}}\limits_{Intense\,blue\,ppt.} + 12KCl}$$
Compound Y on reaction with potassium hexacyanoferrate (III) forms brown colouration due to
$$\mathrm{Fe[Fe(CN)_6]}$$
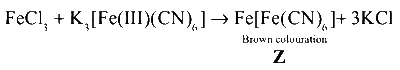
Comments (0)


