JEE Advance - Chemistry (2009 - Paper 1 Offline - No. 11)
The compound(s) that exhibit(s) geometrical isomerism is(are)
$$\mathrm{\left[ {Pt(en)C{l_2}} \right]}$$
$$\mathrm{\left[ {Pt{{(en)}_2}} \right]C{l_2}}$$
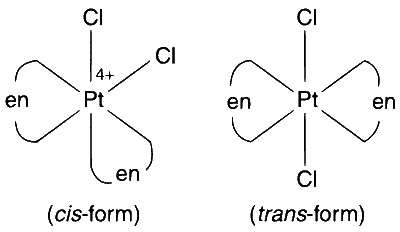
$$\mathrm{\left[ {Pt{{(en)}_2}C{l_2}} \right]C{l_2}}$$
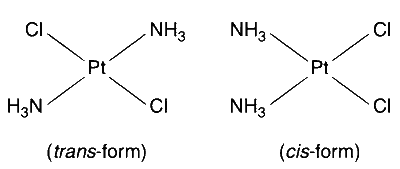
$$\mathrm{\left[ {Pt{{(N{H_3})}_2}C{l_2}} \right]}$$
Explanation
Among the given compounds, the compounds of the type [MA$$_2$$X$$_2$$] and [M(AA)$$_2$$X$$_2$$] will exhibit geometrical isomerism.
Comments (0)


