JEE Advance - Chemistry (2008 - Paper 2 Offline - No. 6)
Explanation
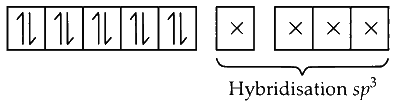
In [Ni(CO)$$_4$$], Ni is in 0 oxidation state, so there are 8 electrons in 3d subshell and 2 electrons in 4s. CO is the strong ligand, so it cause pairing of electrons in 3d subshell leaving one d subshell vacant. Now, the electron from s shell shifts to d creating vacant space in s subshell and thus the hybridisation is sp$$^3$$.
Ni(28) = [Ar] 4s$$^2$$3d$$^8$$
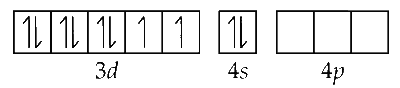
CO is a strong ligand, causes coupling.
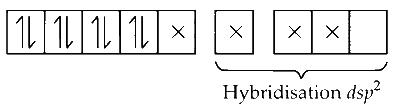
In [Ni(CN)$$_4$$]$$^{2-}$$, Ni is in +2 oxidation state so it has 8 electrons in d subshell and CN is also a strong ligand which pairs with d subshell atom and leaves one d orbital empty and thus its hybridisation is dsp$$^2$$.
In [Ni(CN)$$_4$$]$$^{2-}$$, the oxidation state of Ni is +2
Ni$$^{2+}$$ = [Ar] 3d$$^8$$4s$$^0$$
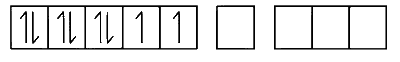
CN$$^-$$ is strong ligand, causes coupling.
Comments (0)


