JEE Advance - Chemistry (2008 - Paper 2 Offline - No. 21)
Match the compounds in Column I with their characteristic test(s)/reaction(s) given in Column II. Indicate your answer by darkening the appropriate bubbles of the 4 $$\times$$ 4 matrix given in the ORS.
| Column I | Column II | ||
|---|---|---|---|
| (A) | 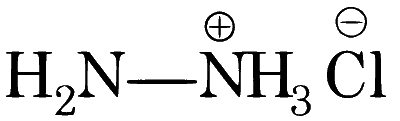 |
(P) | sodium fusion extract of the compound gives Prussian blue colour with FeSO$$_4$$. |
| (B) | 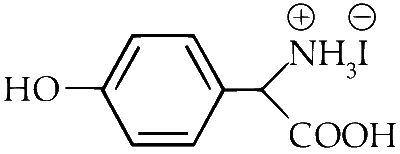 |
(Q) | gives positive FeCl$$_3$$ test. |
| (C) | 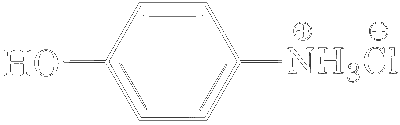 |
(R) | gives white precipitate with AgNO$$_3$$. |
| (D) | 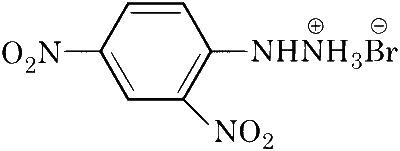 |
(S) | reacts with aldehydes to form the corresponding hydrazone derivative. |
A $$\to$$ (r, s); B $$\to$$ (p); C $$\to$$ (p, q); D $$\to$$ (s)
A $$\to$$ (r, s); B $$\to$$ (p, q); C $$\to$$ (p, q, r); D $$\to$$ (p, s)
A $$\to$$ (r); B $$\to$$ (p, q); C $$\to$$ (p, r); D $$\to$$ (p, s)
A $$\to$$ (r); B $$\to$$ (p); C $$\to$$ (p, q, r); D $$\to$$ (s)
Explanation
A, B, C, D will give NaCN in their sodium fusion extract. Adding FeSO$$_4$$ will give Fe$$_4$$[Fe(CN)$$_6$$]$$_3$$ which is Prussian blue in colour.
B and C have Phenol group which gave positive result to FeCl$$_3$$ test.
A, B, C, D both give precipitate with AgNO$$_3$$ but only A and C will give pure white precipitate.
A and D can only give hydrazone derivative in reaction with aldehyde.
Comments (0)


