JEE Advance - Chemistry (2008 - Paper 2 Offline - No. 2)
The correct stability order for the following species is :
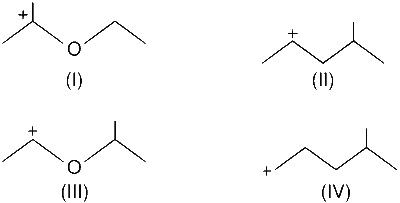
(II) > (IV) > (I) > (III)
(I) > (II) > (III) > (IV)
(II) > (I) > (IV) > (III)
(I) > (III) > (II) > (IV)
Explanation
Stability of the following species depends upon the no. of $$\alpha$$ hydrogen which can undergoes hyperconjugation as well as resonance. Higher the no. of $$\alpha$$ hydrogen, higher will be the stability of the carbocation.
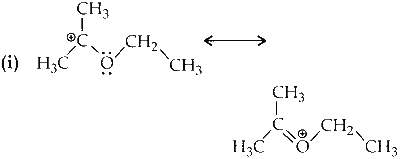
Stabilizes by resonance and have six $$\alpha$$-hydrogen atoms (hyperconjugation).
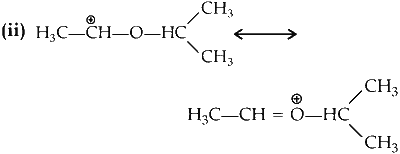
Stabilizes by resonance and have three $$\alpha$$-hydrogen atoms.
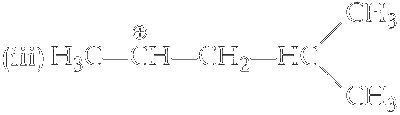
It have five $$\alpha$$-hydrogen atoms.
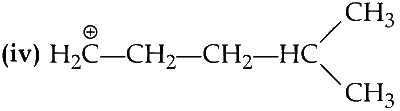
It have only two $$\alpha$$-hydrogen atoms.
$$\therefore$$ (I) > (III) > (II) > (IV)
Comments (0)


