JEE Advance - Chemistry (2008 - Paper 2 Offline - No. 13)
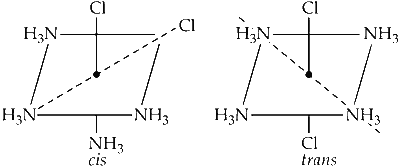
Statement 1 : The geometrical isomers of the complex [M(NH$$_3$$)$$_4$$Cl$$_2$$] are optically inactive.
Statement 2 : Both geometrical isomers of the complex [M(NH$$_3$$)$$_4$$Cl$$_2$$] possess axis of symmetry.
Statement 1 is True, Statement 2 is True; Statement 2 is a CORRECT explanation for Statement 1.
Statement 1 is True, Statement 2 is True; Statement 2 is a NOT CORRECT explanation for Statement 1.
Statement 1 is True, Statement 2 is False.
Statement 1 is False, Statement 2 is True.
Explanation
For a complex to be optically active, it should not possess either centre of symmetry or axis of symmetry or plane of symmetry. Here, the given complex is an octahedral complex that possesses plane of symmetry as well as axis of symmetry and thus is optically inactive.
The $$cis$$ and $$trans$$ both form of complex [M(NH$$_3$$)$$_4$$Cl$$_2$$] are optically inactive.
Comments (0)


