JEE Advance - Chemistry (2008 - Paper 2 Offline - No. 12)
Statement 1 : [Fe(H$$_2$$O)$$_5$$NO]SO$$_4$$ is paramagnetic.
Statement 2 : The Fe in [Fe(H$$_2$$O)$$_5$$NO]SO$$_4$$ has three unpaired electrons.
Statement 1 is True, Statement 2 is True; Statement 2 is a CORRECT explanation for Statement 1.
Statement 1 is True, Statement 2 is True; Statement 2 is a NOT CORRECT explanation for Statement 1.
Statement 1 is True, Statement 2 is False.
Statement 1 is False, Statement 2 is True.
Explanation
The oxidation state of Fe in the complex, [Fe(H$$_2$$O)$$_5$$NO]SO$$_4$$ is +1 [NO has +1 charge]
Fe$$^+$$: [Ar] 3d$$^+$$4s$$^1$$
When the weak ligand H$$_2$$O and strong ligand NO attacks, the configuration changes.
NO$$^+$$ causes pairing of 4s electrons inside. Thus the configuration is 3d$$^7$$ and number of unpaired electrons = 3.
Fe$$^+$$ : [Ar] 3d$$^7$$4s$$^0$$
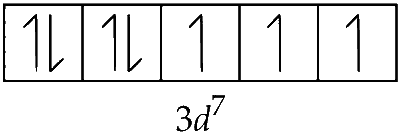
$$\therefore$$ Fe$$^+$$ has 3 unpaired electrons.
Comments (0)


