JEE Advance - Chemistry (2008 - Paper 2 Offline - No. 11)
Statement 1 : Aniline on reaction with NaNO$$_2$$/HCl at 0$$^\circ$$C followed by coupling with $$\beta$$-naphthol gives a dark blue coloured precipitate.
Statement 2 : The colour of the compound formed in the reaction of aniline with NaNO$$_2$$/HCl at 0$$^\circ$$C followed by coupling with $$\beta$$-naphthol is due to the extended conjugation.
Explanation
Let us discuss the given statements one by one.
Statement 1 : Aniline reacts with NaNO$$_2$$/HCl at 273 K to form colourless diazonium salt.
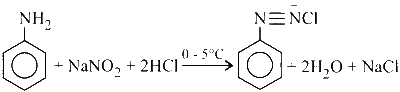
Benzene diazonium chloride is then coupled with $$\beta$$-naphthol to yield a dark red or orange coloured dye as shown in the reaction below:
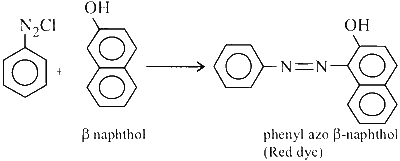
Thus, the statement 1 is false as the colour of the precipitate formed is orange red not blue. As we have already seen in the above mentioned reactions that the colour compound (dye) formed in the reaction of aniline with NaNO$$_2$$/HCl at 0$$^\circ$$C was due to the successive coupling of diazonium salt with $$\beta$$-naphthol. Hence, statement 2 is true.
As a result, Statement 1 is False and Statement 2 is true.
Comments (0)


