JEE Advance - Chemistry (2008 - Paper 1 Offline - No. 8)
The correct statement(s) about the compound given below is(are):
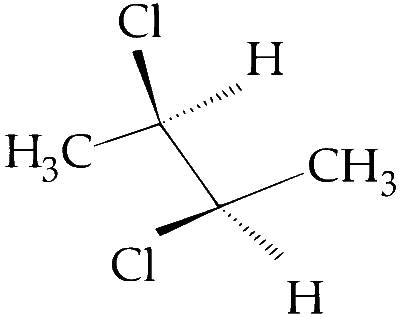
The compound is optically active.
The compound possesses centre of symmetry.
The compound possesses plane of symmetry.
The compound possesses axis of symmetry.
Explanation
We know that following are the cases when a compound is optically inactive:
1. When it does not have a chiral carbon atom e.g., Methyl bromide
2. When it has a plane or axis of symmetry e.g., Tartaric acid (these compounds are called as Meso compounds)
3. When it forms a racemic mixture e.g.: Equimolar mixture of d-Lactic acid and l-Lactic acid.
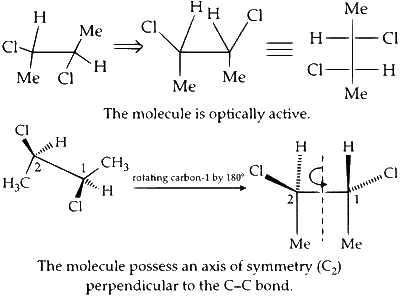
The given compound possesses an axis of symmetry
Comments (0)


