JEE Advance - Chemistry (2008 - Paper 1 Offline - No. 3)
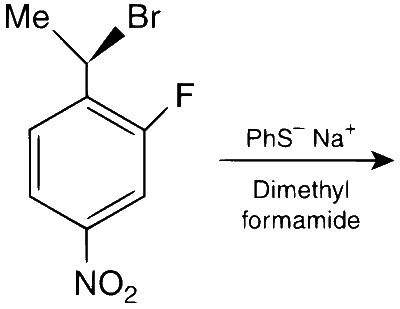
The major product of the following reaction is:
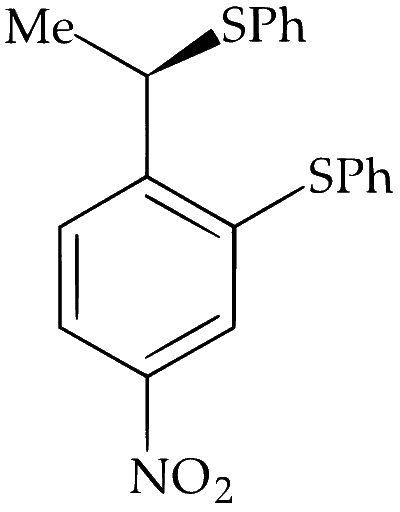
Explanation
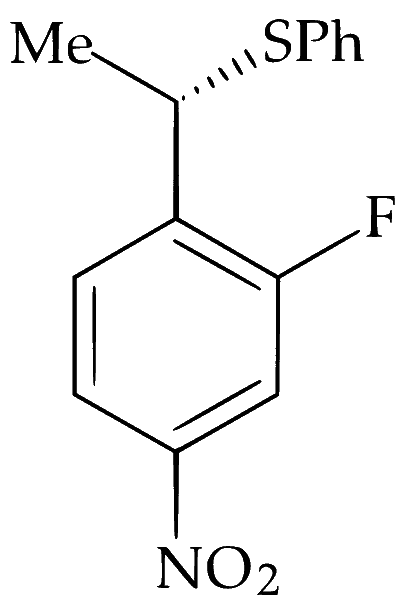
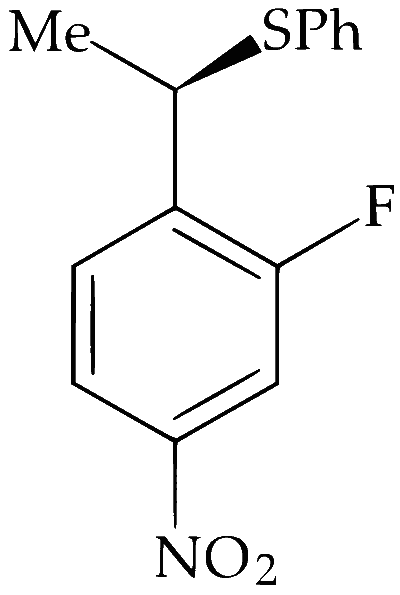
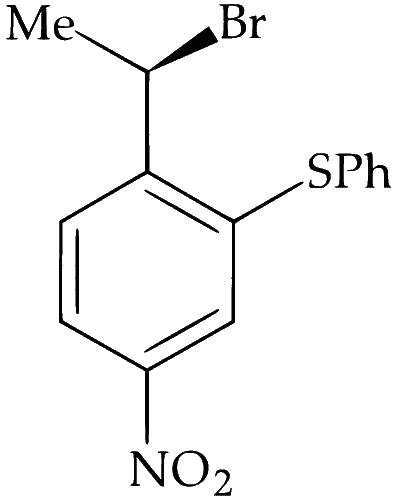
Alkyl halides undergo nucleophilic substitution more readily than aryl halides due to partial double bond character between aryl carbon and halogen. Hence, substitution occurs more readily in the side chain rather than in the ring. PhS$$^-$$ is a strong nucleophile and dimethylformamide (DMF) is a highly polar aprotic solvent. Hence, nucleophilic substitution (S$$_\mathrm{N}2$$) takes place at 2$$^\circ$$ benzylic carbon. Stereochemically, it involves inversion of configuration at benzylic carbon atom.
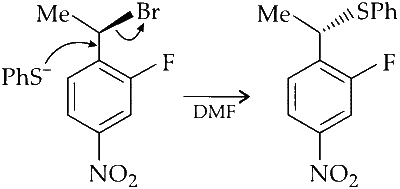
Alkyl group exhibit neighbouring group participation or anchimeric assistance from aryl group but deactivation by $$-$$NO$$_2$$ and $$-$$F makes S$$_\mathrm{N}1$$ difficult and retention of configuration at chiral carbon does not takes place.
Comments (0)


