JEE Advance - Chemistry (2008 - Paper 1 Offline - No. 12)
Statement 1 : Bromobenzene upon reaction with Br$$_2$$/Fe gives 1, 4-dibromobenzene as the major product.
and
Statement 2: In bromobenzene, the inductive effect of the bromo group is more dominant than the mesomeric effect in directing the incoming electrophile.
Statement 1 is True, Statement 2 is True; Statement 2 is a correct explanation for Statement 1.
Statement 1 is True, Statement 2 is True; Statement 2 is a NOT correct explanation for Statement 1.
Statement 1 is True, Statement 2 is False.
Statement 1 is False, Statement 2 is True.
Explanation
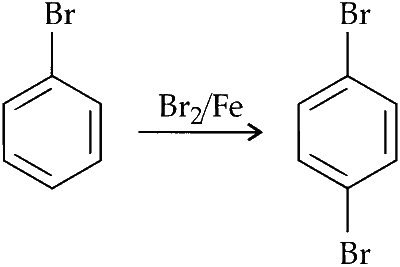
Bromine orientation is controlled by its mesomeric effect. Its stabilisation of arenium ion by +M activity.
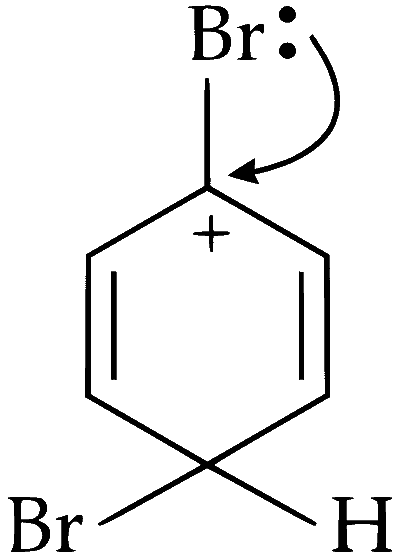
In bromobenzene, the inductive effect of the bromo group is more dominant than the mesomeric effect in directing the incoming electrophile at para position. As a result, only mono substitution of bromobenzene takes place.
Comments (0)


