JEE Advance - Chemistry (2008 - Paper 1 Offline - No. 1)
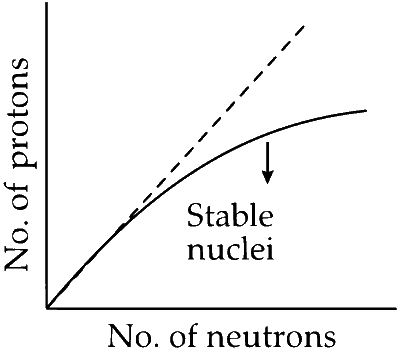
STATEMENT - 1 : The plot of atomic number (y-axis) versus number of neutrons (x-axis) for stable nuclei shows a curvature towards x-axis from the line of 45o slope as the atomic number is increased.
STATEMENT - 2 : Proton-proton electrostatic repulsions begin to overcome attractive forces involving protons and neutrons in heavier nuclides
STATEMENT - 2 : Proton-proton electrostatic repulsions begin to overcome attractive forces involving protons and neutrons in heavier nuclides
Statement - 1 is True, Statement - 2 is True; Statement - 2 is a correct explanation for Statement - 1
Statement - 1 is True, Statement - 2 is True; Statement - 2 is NOT a correct explanation for Statement - 1
Statement - 1 is true, Statement - 2 is False
Statement - 1 is False, Statement - 2 is True
Explanation
With increase in the atomic number, the proton-proton electrostatic repulsion begins to overcome attractive forces involving proton and neutrons in heavier nuclides. The stability relationship can be represented by a line with a slope of 45$$^\circ$$, i.e., the maximum stability is attained when N = Z. Right of the curve a radioactive nuclide would be neutron rich and would decay by $$\beta$$-emission to produce a daughter nucleus with a lower n/p ratio. For heavier nuclides, p-p repulsions start to offset the attractive forces and an excess of neutrons over protons, is required for stability.
Comments (0)


