JEE Advance - Chemistry (2007 - Paper 2 Offline - No. 2)
This question contains STATEMENT-1 (Assertion) and STATEMENT-2 (Reason) and has 4 choices (a), (b), (c), (d) out of which ONLY ONE is correct.
STATEMENT-1 : Band gap in germanium is small. because
STATEMENT-2 : The energy gap of each germanium atomic energy level is infinitesimally small.
STATEMENT-1 : Band gap in germanium is small. because
STATEMENT-2 : The energy gap of each germanium atomic energy level is infinitesimally small.
Statement - 1 is True, Statement - 2 is True; Statement - 2 is a correct explanation for Statement - 1
Statement - 1 is True, Statement - 2 is True; Statement - 2 is not a correct explanation for Statement - 1
Statement - 1 is True, Statement - 2 is False
Statement - 1 is False, Statement - 2 is True
Explanation
A bond gap is the difference between conduction band and valence band. In germanium band gap is 64 kJ/mol. As Ge is semiconductor so, band gap in germanium is small.
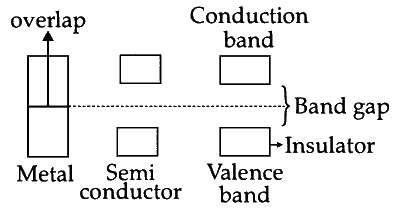
On moving top to bottom in the group 14 band gap decreases.
Statement- 1 and 2 both are correct but Statement- 2 is not correct explanation of Statement- 1.
Comments (0)


