JEE Advance - Chemistry (2005 - No. 1)
At constant temperature and volume, X decomposes as 2X(g) $$\to$$ 3Y(g) + 2Z(g); Px is the partial pressure of X.
(i) What is the order of the reaction to X?
(ii) Find the rate constant
(iii) Find the time for 75% completion of the reaction.
(iv) Find the total pressure when pressure of X is 700 mm of Hg
| Observation No. | Time (in minute) | Px (in mm of Hg) |
|---|---|---|
| 1 | 0 | 800 |
| 2 | 100 | 400 |
| 3 | 200 | 200 |
(i) What is the order of the reaction to X?
(ii) Find the rate constant
(iii) Find the time for 75% completion of the reaction.
(iv) Find the total pressure when pressure of X is 700 mm of Hg
First order, $$6.93 \times 10^{-3}$$ min$$-1$$, 200 min, 950 mm Hg
Second order, $$3.465 \times 10^{-3}$$ min$$-1$$, 100 min, 850 mm Hg
Zero order, $$6.93 \times 10^{-3}$$ min$$-1$$, 50 min, 900 mm Hg
First order, $$3.465 \times 10^{-3}$$ min$$-1$$, 200 min, 950 mm Hg
First order, $$6.93 \times 10^{-3}$$ min$$-1$$, 100 min, 850 mm Hg
Explanation
The given reaction is : 2X(g) $$\to$$ 3Y(g) + 2Z(g)
(1) Partial pressure (of X) trend :
800 mm Hg $$\buildrel {100\,\min } \over \longrightarrow $$ 400 mm Hg $$\buildrel {100\,\min } \over \longrightarrow $$ 200 mm Hg Half-life is constant, hence, it is a first order reaction.
(2) For a first order reaction, rate constant $$k = {{0.693} \over {half - life}}$$
or, $$k = {{0.693} \over {100}} = 6.93 \times {10^{ - 3}}$$ min$$-$$1
(3) Time required for the completion of 75% of the reaction is equal to 2 half lives. Hence, Time taken = (2 $$\times$$ 100) = 200 min
(4) 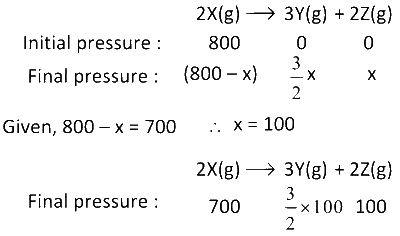
$$\therefore$$ Total pressure = (700 + 150 + 100) mm Hg = 950 mm Hg
Comments (0)


