JEE Advance - Chemistry (2004 - No. 5)
Draw the structure of XeF4 and OSF4 according to VSEPR theory clearly indicating the state of hybridisation of the central atom and lone pair of electrons (if any) on the central atom.
XeF4: sp3d2, square planar, 2 lone pairs; OSF4: sp3d, distorted trigonal bipyramidal, 0 lone pairs
XeF4: sp3d, square planar, 1 lone pair; OSF4: sp3d2, trigonal bipyramidal, 1 lone pair
XeF4: sp3, tetrahedral, 0 lone pairs; OSF4: sp3, tetrahedral, 0 lone pairs
XeF4: sp3d2, octahedral, 2 lone pairs; OSF4: sp3d, trigonal planar, 1 lone pair
XeF4: sp3d, tetrahedral, 1 lone pair; OSF4: sp3, bent, 0 lone pairs
Explanation
Structure of Xenon tetrafluoride, $\mathrm{XeF}_4$ :
In $\mathrm{XeF}_4$, the central atom $\mathrm{Xe}$ is attached to $4 \mathrm{~F}$ atoms. The central atom $\mathrm{Xe}$ undergoes $s p^3 d^2$ hybridization as shown below :
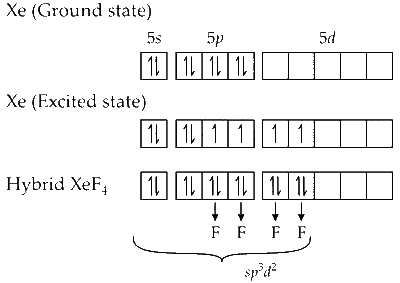
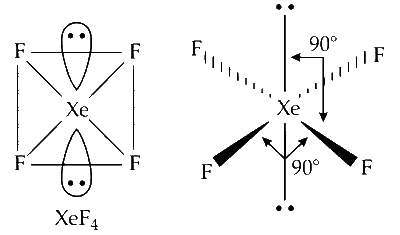
The geometry of $\mathrm{XeF}_4$ is square planar and the structure is octahedral. The bond angle is $90^{\circ}$. The two lone pairs of electrons are shown on $\mathrm{Xe}$ atom.
Structure of Thionyl Tetrafluoride, $\mathrm{OSF}_4$
In $\mathrm{OSF}_4$, the central atom is sulphur which is attached to a $\mathrm{O}$ atom by double bond and attached to $4 \mathrm{~F}$ atoms through a single bond. The central $S$ atom undergoes $s p^3 d$ hybridisation as shown below :
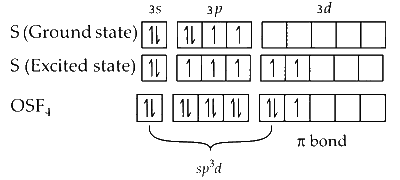
One of the $d$ orbitals will be involved in pi bond formation with $\mathrm{O}$ atom.
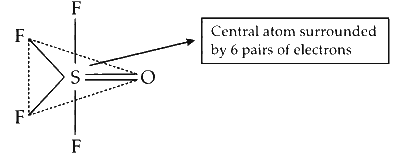
Thus, the molecular geometry of $\mathrm{OSF}_4$ should be trigonal bipyramidal. The geometry is distorted trigonal bipyramidal due to asymmetric charge distribution around the central atom. In the molecule, zero lone pairs are present along with 5 sigma bonds and a pi bond. The oxygen atom is at equatorial position as it is less electronegative than that of $\mathrm{F}$.
Comments (0)


