JEE Advance - Chemistry (2002 - No. 3)
Specify the coordination geometry around and hybridisation of N and B atoms in a 1 : 1 complex of BF3 and NH3
N : tetrahedral, sp3; B : tetrahedral, sp3
N : pyramidal, sp3; B : pyramidal, sp3
N : pyramidal, sp3; B : planar, sp2
N : pyramidal, sp3; B : tetrahedral, sp3
Explanation
The correct answer is Option A: N: tetrahedral, sp³; B: tetrahedral, sp³
Explanation:- BF₃ (Boron trifluoride): In its isolated state, BF₃ has a trigonal planar geometry around the boron atom with sp² hybridization.
- NH₃ (Ammonia): In its isolated state, NH₃ has a trigonal pyramidal geometry around the nitrogen atom with sp³ hybridization.
However, when BF₃ and NH₃ form a 1:1 complex, the lone pair of electrons on nitrogen donates to the empty p-orbital of boron. This leads to the formation of a coordinate bond between nitrogen and boron.
In the BF₃-NH₃ complex: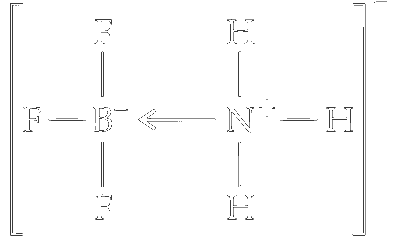
- Nitrogen (N): Now has four bonding pairs of electrons (three with hydrogen and one with boron), resulting in a tetrahedral geometry and sp³ hybridization.
- Boron (B): Also has four bonding pairs of electrons (three with fluorine and one with nitrogen), leading to a tetrahedral geometry and sp³ hybridization.
Key point: The formation of the coordinate bond between nitrogen and boron changes the hybridization and geometry of both atoms in the complex.
Comments (0)


