JEE Advance - Chemistry (1999 - No. 9)
The geometry of H2S and its dipole moment are
angular and non-zero
angular and zero
linear and non-zero
linear and zero
Explanation
$\mathrm{H}_2 \mathrm{~S}$ has $s p^3$ hybridised sulphur, therefore, angular in shape with non-zero dipole moment.
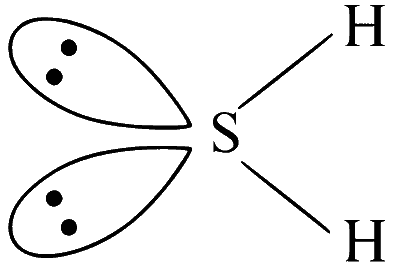
(Non-linear, polar molecule)

(Non-linear, polar molecule)
Comments (0)


