JEE Advance - Chemistry (1999 - No. 13)
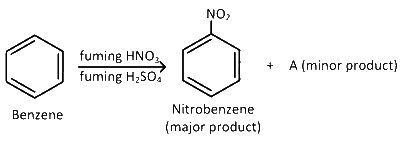
Nitrobenzene is formed as the major product along with a minor product in the reaction of benzene with a hot mixture of nitric acid and sulphuric acid. The minor product consists of carbon : 42.86 %, hydrogen : 2.40%, nitrogen : 16.67% and oxygen : 38.07% (i) Calculate the empirical formula of the minor product. (ii) When 5.5 g of the minor product is dissolved in 45 g of benzene, the boiling point of the solution is 1.84 oC higher than that of pure benzene. Calculate the molar mass of the minor product and determine its molecular and structural formula. (Molal boiling point elevation constant of benzene is 2.53 K kg mol-1)
84 g, C3H2NO2, o-nitrobenzene
168 g, C6H4N2O4, m-dinitrobenzene
168 g, C6H4(NO2)2, p-dinitrobenzene
84 g, C3H2NO2, m-nitrobenzene
168 g, C6H4(NO2)2, m-dinitrobenzene
Explanation
As given in the question,
Determination of empirical formula of A :
| Element with atomic number | Percentage | Relative Number | Simplest ratio |
|---|---|---|---|
| C (12) | 42.86 | $${{42.86} \over {12}} = 3.57$$ | $${{3.57} \over {1.19}} = 3.00$$ |
| H (1) | 2.40 | $${{2.40} \over 1} = 2.40$$ | $${{2.40} \over {1.19}} = 2.00$$ |
| N (14) | 16.67 | $${{16.67} \over {14}} = 1.19$$ | $${{1.19} \over {1.19}} = 1.00$$ |
| O (16) | 38.07 | $${{38.07} \over {16}} = 2.37$$ | $${{2.37} \over {1.19}} = 2.00$$ |
$$\therefore$$ Empirical formula of minor product (A) = C3H2NO2
Empirical formula mass = (12 $$\times$$ 3) + (1 $$\times$$ 2) + 14 $$\times$$ (16 $$\times$$ 2) = 84
Now, $$\Delta$$TB = kb $$\times$$ molality
or, $$1.84 = 2.53 \times {{5.5} \over M} \times {1 \over {45}} \times 1000$$
or, molar mass (M) = $${{2.53 \times 5.5 \times 1000} \over {1.84 \times 45}}$$
$$\therefore$$ M = 168
$$\therefore$$ $${{molar\,mass} \over {formula\,mass}} = {{168} \over {84}} = 2$$
$$\therefore$$ Molecular formula of (A) = (C3H2NO2)2 = C6H4N2O4
Structural formula :
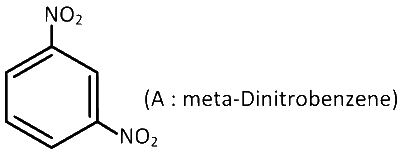
Comments (0)


