JEE Advance - Chemistry (1994 - No. 15)
A is binary compound of a univalent metal. 1.422 g of A
reacts completely with 0.321 g of sulphur in an evacuated
and sealed tube to give 1.743 g of a white crystalline solid
B, that forms a hydrated double salt, C with Al2
(SO4)3
.
Identify A, B and C.
A = KO, B = KSO, C = KSO·Al(SO)·24HO
A = KO, B = KSO, C = KSO·Al(SO)·12HO
A = KO2, B = K2SO4, C = K2SO4·Al2(SO4)3·12HO
A = KO2, B = K2SO4, C = K2SO4·Al2(SO4)3·24H2O
A = KO2, B = K2S, C = K2S·Al2(S)3·24H2O
Explanation
Univalent metal means alkali metal. A is binary compound so A can be KO or KO2.
But here A react completely with sulphur.
$$\therefore$$ A must be KO2.
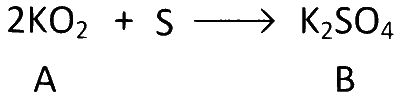
To check A and B are right or not we have to check with their molar ratio.
Here molar ratio of KO2 and S = 2 : 1
Moles of 1.422 g KO2 = $${{1.422} \over {71}} = 0.02$$
Moles of 0.321 g S = $${{0.321} \over {32}} = 0.01$$
$$\therefore$$ Molar ratio = 0.02 : 0.01 = 2 : 1
$$\therefore$$ A and B are right choice.
When K+ is the monovalent cation and Al+3 is the trivalent cation then double salt C is $$ = {K_2}S{O_4}.A{l_2}{(S{O_4})_3}.24{H_2}O$$
Comments (0)


